ORIGINAL
Cellulolytic and Butyrivibrio fibrisolvens bacteria population density, after supplementing fodder diets (Pennisetum clandestinum)
Densidad poblacional de bacterias celulolíticas y de Butyrivibrio fibrisolvens al suplementar dietas forrajeras (Pennisetum clandestinum)
Licet Molina G,1 M.Sc, Luis Giraldo V, 2 P.hD, Diana Polanco E, 1 M.Sc, Lina Gutiérrez B, 1,3* Ph.D.
1Universidad de Antioquia, Escuela de Microbiología, Grupo de Investigación en Microbiología Veterinaria, Calle 67 # 53-108, AA 1226. Medellín, Colombia.
2Universidad Nacional de Colombia, Facultad de Ciencias Agrarias, Departamento de Producción Animal, Grupo Biotecnología Ruminal y Silvopastoreo “BIORUM”, Calle 59A No 63-20. Medellín, Colombia.
3Escuela de Ciencias de la salud, Facultad de Medicina; Universidad Pontificia Bolivariana; Sede Central Medellín; Calle 78B N°72A-109, Medellín, Colombia.
*Correspondence: liangutibui@gmail.com
Received: April 2014; Acepted: February 2015.
ABSTRACT
Objective. Determine the population density of cellulolytic bacteria, Butyrivibrio fibrisolvens and the concentration of vaccenic acid, by supplementing diets consisting of kikuyu grass (Pennisetum clandestinum Hoechst. Ex Chiov.) as base ingredient, together with cassava flour and biomass (effluent from ethanol production) in rumen simulator-Rusitec. Materials and methods. Four treatments (T) were evaluated, these were composed as: T1/Control 1: 100% kikuyu grass with a total protein intake of 23.9%, T2: a mixture of 70% kikuyu grass, 20% biomass and 10% cassava flour with a total protein intake of 19.4%; T3/Control 2: 100% kikuyu grass, with a 17.8% protein intake and T4: 70% kikuyu grass, 20% biomass and 10% cassava flour with a 15.3% protein intake. One and two-way variance analysis was made and the Pearson correlation coefficient was determined. Results. An increase was observed in the population density of viable cellulolytic bacteria (CFU/ml) and B. fibrisolvens statistically significant (p<0.005) with treatment T2, in contrast to T1, T3 and T4 treatments. In addition, there was a significant increase in the concentration of vaccenic acid (mg/L) in the ruminal content in Rusitec with the same treatment (T2). Conclusions. Results obtained in this ruminal simulation study are evidence to the benefits of kikuyu grass together with cassava flour and biomass diet implementation on the growth of ruminal cellulolytic and B. fibrisolvens bacteria, as well as on the production of vaccenic acid. The study also suggests the nutritional potential that such supplements could provide to grazing bovine feeding.
Key words: Dietary supplements, fermentation, ruminal fermentation, Rumen (Source: MeSH).
RESUMEN
Objetivo. Determinar la densidad poblacional de bacterias celulolíticas, Butyrivibrio fibrisolvens y la concentración de ácido vaccénico, al suplementar dietas forrajeras de pasto kikuyo con harina de yuca y biomasa (resultante de la producción de etanol), en el simulador de rumen- Rusitec. Materiales y métodos. Se evaluaron cuatro tratamientos (T): T1/Control 1: 100% pasto kikuyo con un aporte total de 23.9% de proteína, T2: 70% pasto kikuyo, 20% biomasa y 10% harina de yuca con un aporte total de 19.4% proteína, T3/Control 2: 100% pasto kikuyo con un aporte total de 17.8% de proteína y T4: 70% pasto kikuyo, 20% biomasa y 10% harina de yuca con un aporte total de 15,3% de proteína. Se realizó un análisis de varianza (ANOVA) de una y dos vías y se determinó el coeficiente de correlación de Pearson. Resultados. Se observó un incremento en la densidad poblacional de bacterias celulolíticas viables (UFC/ml) y de B. fibrisolvens estadísticamente significativas (p<0.005) con el tratamiento T2, en comparación con los tratamientos T1, T3 y T4. Adicionalmente, se detectó un aumento significativo en la concentración de ácido vaccénico (mg/L) en el contenido ruminal del Rusitec con el mismo tratamiento (T2). Conclusiones. Los resultados obtenidos en este estudio de simulación ruminal indican que la suplementación del pasto kikuyo con harina de yuca y biomasa favorecen el crecimiento de las bacterias celulolíticas ruminales y de B. fibrisolvens, así como la producción de ácido vaccénico, y sugieren el potencial nutricional que podría tener este tipo de suplementación en la alimentación de bovinos en pastoreo.
Palabras clave: Fermentación, fermentación ruminal, Rumen, Suplementos dietéticos (Fuente: MeSH).
INTRODUCTION
Cattle production in tropical areas like Colombia depends mainly on fodder supply from the pastures. Degradation of lignocellulosic matter contained in fodder is carried out by rumen microbial consortia, specifically by cellulolytic bacteria that take parton the degradation of structural carbohydrates to volatile fatty acids assimilated by the ruminant (1-3).
Butyrivibrio fibrisolvens is a strict ruminal anaerobe, with high cellulolytic, hemicellulolytic, proteolytic and uricolytic activity. It may represents significant portion (10-30%) of culture ruminal bacteria (4). This bacteria plays a major role in fiber degradation and it has been identified as the main bacterial species that participates in the process of biohydrogenation on unsaturated fatty acids present in diets based on fodder, grains and oils (5), which is a process that could lead to an increase in the concentration of fatty acids in milk and meat from ruminants (6,7).
Some pastures available in different regions in Colombia lack adequate nutritional quality, and its production are seasonal, which reduces quality and return in the industry of milk and meat cattle (8). Previous research has suggested that supplementation of diets different industry subderivados (maize, cassava, fish oil, sunflower oil, etc.) improves use of high-fiber content and low digestibility fodder, enhance biodiversity of ruminal microorganisms and ruminal fermentation processes and, consequently, animal productivity (5,7). In the search of food supplements that improve digestion and degradation processes of fodders by bovines, diverse alternatives to pastures supplementation, mainly with fermentable and additive carbohydrates such as oils, concentrated food and grains, have been proposed.
In 2008, and with the support of the Ministry of Agriculture and Rural Development of Colombia, the National Fuel Alcohol Program was created. One of the purposes of the program was to evaluate the production of ethanol from sugar cane and other agricultural sources such as banana, cassava and sorghum, with the objective of improving quality of fuels by biologic oxygenation and substitution of compounds such as methyl-tert-butyl ether, due to soil and underground water contamination originated in its use (9-11). Cassava (Manihot esculenta Crantz) has been considered one of the viable alternatives for ethanol production in the country, that together with corn, sugar cane and rice, are considered the most important energy sources in tropical regions worldwide, as it becomes one of the alternative crops with highest potential for ethanol production (12,13). Cassava has been widely considered a basic energetic food item in the industry of balanced food for animals, because of its protein and starch content. In another strategy, the use of cassava flour together with the biomass originated in ethanol production might constitute a promising supplementation practice for pastures in which yields are insufficient to achieve acceptable levels of production. It might also be considered an additional source of protein and energy for bovines (13).
Kikuyu grass (Pennisetum clandestinum Hoechst. Ex Chiov.) is considered a basic feeding element by specialized dairy production systems in Colombia (14). Considering that fodder quality decreases as the plant grow solder, fibrous portion increases and decreases the protein content, digestibility and availability of energy for the animal lowered, which makes the evaluation of different alternatives of energetic supplementation to be included in the diet of the animal (13), it is possible that supplementing fodder such as kikuyu, could modify the ruminal biohydrogenation process, together with the sub-sequent formation of the principal components of CLA: rumenic acid (cis-9,trans-11) and vaccenic acid (trans-11C18:1).
This study takes into consideration the participation of ruminal cellulolytic bacteria in fiber degradation and the participation of B. fibrisolvens in the process of biohydrogenation for CLA production as important indicators of ruminal fermentation. In this regard, the objective of this study was to determine the population density of the cellulolytic bacteria, B. fibrisolvens and the concentration of vaccenic acid, in response to the use of cassava flour and effluent biomass from ethanol production as supplements to Kikuyu grass with different concentration of protein in an artificial rumen (Rusitec).
MATERIALS AND METHODS
Ethical aspects of the study. This study was approved by the Ethics Committee on Animal Experimentation from the National University of Colombia, Medellín site, in accordance to Deed CEMED-027, issued on December 2nd, 2008.
Type of study. Comparative experimental design with four treatments.
Ruminal microorganisms’s inoculum used in Rusitec Fermenters. To start the ruminal fermentation simulation (Rusitec), ruminal content from four female Holstein cows (700±25 kg live weight) was used as initial inoculum. The cows were rumen-fistulated and kept under a basal diet of kikuyu grass in the Paysandú farm, owned by the National University of Colombia, Medellín site, and located in the town of Santa Elena, at 2500 m.a.s.l, with an average temperature of 14°C (15). Rusitec is composed of eight sequential vessels (fermentation units) with a maximum capacity of 700 ml (16). In accordance to the protocols proposed by Czerkawski and Breckenridge (17), the simulated ruminal fermentation experiments were performed in the laboratory of Ruminal Biotechnology at the National University.
The experiment was started by placing, in each vessel, 200 ml of artificial saliva (pH= 8.4; 9.8g of NaHCO3; 3.72g of Na2HPO4; 0.47g of NaCl; 0.57g of KCl; 0.053g of CaCl22H2O and 0.128g of MgCl26H2O, of each component, per liter), 500 ml of ruminal fluid and a solid portion of ruminal content (80g). Such fractions were obtained separately after filtration through four layers of muslin, to ensure the inclusion of present microorganisms in both the liquid and solid fractions of ruminal content. The experiment was carried out at 39°C constant temperature. The simulated fermentation procedure was performed in two periods of seven days corresponding to a four-day adaptation period and a three-day measurement period (5th -7th day of simulation).
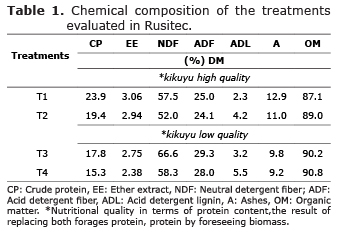
Treatments to be assessed through Rusitec. Kikuyu grass was the forage species selected to assess the effect of supplementation with cassava flour and biomass resulting from ethanol production, on cellulolytic and B. fibrisolvens bacteria, in Rusitec. Kikuyu grass was gathered in the municipality of San Pedro de los Milagros, with a 35-day regrowth age, while using nitrogen fertilization with urea (N:50-70, P2O5:45.8, K2O:18, MgO:24.75, SO4:44.85). Two types of kikuyu grass good and bad quality with contrasting nutritional quality in terms of protein content, the result of replacing both forages protein, protein by foreseeing biomass, were included to evaluate the response to biomass and cassava flour as dietary supplements. The amount of kikuyu grass, plus the addition of substrate evaluated in each vessel, was 18g (10.3-15.5g of dry matter). The grass, with a particle size of 1.5 mm, was stored in nylon bags with a pore size of 45 µm. To avoid dilution of the products resulting from the fermentation system, a dilution rate of 3.5% per hour was established.
The assessment of treatments through Rusitec was carried out as follows: On day zero, the experiment started with a test performed in the Rusitec fermenter in eight fermenting pots (2 crocks per treatment). Once the ruminal liquid and artificial saliva were poured into each crock, two nylon bags, containing 18g of the treatment to be assessed and 80g of ruminal content respectively, were introduced in the containers. This last bag was removed 48 hours after incubation initiated. During the following days (1st-7th), each bag in each fermenter was replaced by a new substrate bag every 24 hours. During the 5th, 6th and 7th days, the ruminal liquid was collected from each fermenting crock, before replacing the bag containing the substrate, in order to perform the quantification of ruminal microorganisms, the determination of vaccenic acid and the immediate pH measurement.
Two duplicates of the simulation experiment were carried out in two different moments, with a 15-day interval between them, but under the same conditions. This was aimed at estimating more accurately the effect of the supplements to kikuyu on the microbial population to be evaluated. The assessed treatments (T) were: T1/Control 1: 100% kikuyu grass with a total protein intake of 23.9%; T2: a mixture of 70% kikuyu grass, 20% biomass and 10% cassava flour with a total protein intake of 19.4%; T3/Control 2: 100% kikuyu grass, with a 17.8% protein intake; and T4: 70% kikuyu grass, 20% biomass and 10% cassava flour with a 15.3% protein intake (Tables 1 and 2).
Sampling procedure. Thirty five milliliters of the ruminal content mixture collected from the four Holsteins were extracted to perform the isolation of native strains of B. fibrisolvens and determine the initial quantity of cellulolytic and B. fibrisolvens bacteria and the initial concentration of vaccenic acid, contained in the initial inoculum. After the adaptation period to the system, during the three days of sampling, 2 aliquots of ruminal content were extracted from each fermenter, every 24 hours: 15 ml were employed to measure the pH and complete the procedures of cellulolytic bacteria quantification; 20 ml, which were stored at -20°C, were later used for DNA extraction and B. fibrisolvens real-time qPCR quantification. In addition, to measure vaccenic fatty acid through gas chromatography, for this process were took between 0.15 to 0.20 g sample, 4.0 ml of 0.5 N NaOH was added in methanol and placed in a water bath at 90°C, the system was allowed to reflux for 7 minutes elapsed time reflux, 5 ml of buffer F3 was added to 12% methanol by the top of the condenser and allowed to reflux for 2 minutes. Subsequently 4.0 ml of N-heptane was added to the top of the condenser and was refluxed for 1 minute, cooled and 100 ml of saturated NaCl to ensure the extraction of the compounds separated in two phases solution is added.
The organic phase was removed with a Pasteur pipette into a test tube and added anhydrous sodium sulfate to remove moisture was used, in a column: DB–23, capillary of 50.0 m, inside diameter 0.25 mm, film thickness of 1.4 µm, detector temperature of 280 °C and injector temperature of 250°C, hydrogen injection (carrier gas): 40 m/seg, H2 = 45 ml/min flow detector and 450 ml/min airflow (Agilent 6890 Series Gas Chromatograph, Wilmington, DE).
Viable cellulolytic bacteria (VCB) quantification. Serial dilutions (until 10-6) in triplicate, using solvent (phosphate and mineral salts, resazurin, calcium carbonate and L-cysteine-HCL) were carried out using 1ml of the sample extracted from the initial inoculum and each fermenter, during sample period. One milliliter from dilutions 10-3 to 10-6 was extracted and added to solid pre-reduced media prepared by means of Roll-tube technique (18). These culture media contained rumen fluid clarified at 40%, cellobiose at 0.1% and yeast extract at 0.25%, together with other components recommended by Grubb and Dehoroty (19). In order to prepare the solid medium film in culture tubes, agar was added to a final concentration of 1.5% and the culture was grown in anaerobic conditions, by inoculating the hermetically closed sample, through the rubber lid of the tube and using a syringe containing N2. The colony count was performed after 72 hours of incubation at 39°C and the results were given in terms of Colony Forming Units (CFU/ml) (18).
qPCR of B. fibrisolvens. The standard curve for the quantification of B. fibrisolvens through qPCR was performed with the reference strain ATCC #19171, stored in axenic culture (108cells/ml). The number of cells in the culture after 24 hours was calculated at a wavelength of 640 nm. One milliliter was extracted from the culture for centrifugation and DNA extraction with DNeasy® Blood & Tissue commercial kit (QIAGEN, MD, USA). The serial dilution (10-1-10-6) was performed for the DNA extracted from the reference strain. Such dilutions were used to perform the specific standard curve for the qPCR of B. fibrisolvens in the samples of rumen obtained from the Rusitec. The curve was generated by considering the values of the cycle threshold (Ct), observed in 35 cycles, versus the logarithm of the number of cells/ml, starting with the six dilutions of pure culture DNA of well-known concentration. The qPCR protocol followed in this study was validated by the analysis of two internal controls, genomic DNA from the reference strain ATCC #19171 and DNA from the reference strain ATCC #19171 added to 1 ml of ruminal content and subjected to extraction protocol. The amplification of both controls was compared in separate and multiple reactions, to determine the efficiency and specificity of the method and to discard the presence of inhibitors in the ruminal content samples. Genomic DNA extraction was performed from the samples stored at -20°C, collected from the initial inoculum and from each fermenter in the Rusitec during the sampling period. Quality and concentration of the extracted DNA was assessed using NanoDrop ND1000 Spectrophotomer (Thermo Scientific, Wilmington, DE) and 80 ng from the DNA extracted from each sample were analyzed.
The qPCR was performed in Applied Biosystems 7500 Fast Real-Time PCR system (Applied Biosystems, CA, USA). The amplification reactions were carried out in a final volume of 25µl that contained 12.5 µl of SYBR® Green (QIAGEN, MD, USA) and 0.5 µM of each primer for the ARNr 16S gene amplification. After a 15 minute denaturation at 95°C, the amplification and quantification program was repeated 35 times (30s at 94°C; 30s at 55°C and 30s at 72°C), followed by the fusion curve analysis (46°C-95°C, with temperature increase of 3°C per minute and continuous measurement of fluorescence).
The Ct value was calculated automatically by the equipment and the amplification of sample was considered negative when the Ct value was lower than 8 and higher than 35, or when an amplification curve was not obtained. The amplification reaction was repeated for all samples with Ct values higher to 35. Each amplification protocol included a negative control (without template DNA), six positive controls in triplicate corresponding to the know concentration DNA (from the standard curve) and the DNA from the internal controls.
The results were reported considering the number of cells/ml. Q-PCR Efficiency (E) was calculated from the slope during the exponential phase, in each cycle according to the equation E=10(- 1/slope). The products of the resulting qPCR amplification were verified by agarose gel electrophoresis to 1%, and dyed with EZvision™Dye (AMRESCO, OH, USA).
Statistical analysis. The data the population density of cellulolytic (CFU/ml) and B. fibrisolvens bacteria (cells/ml) during the sampling period, in response to cassava flour and biomass effluent from ethanol product being added as supplements to kikuyu grass in Rusitec, were examined in a one and two-way variance analysis (ANOVA), having complied with the conditions of bivariate normality and homoscedasticity. In addition, an analysis of repeated measures in time was carried out following the Friedman and Wilcoxon tests. Also the Pearson correlation coefficient was determined between the vaccenic acid concentration and the population density of B. fibrisolvens, by means of the Statistical Package for the Social Sciences for Windows software SPSS version 21 (IBM SPSS, Armonk, NY: IBM Corp.).
RESULTS
Population density of viable cellulolytic bacteria and pH in the in Rusitec. The amount of CFU/ml obtained from the initial inoculum, used to start the ruminal fermentation simulator was 7.5x105 CFU/ml. The analysis the CFU/ml amount of cellulolytic bacteria, among the different treatments, showed statistically significant differences (p=0.03). The growth of microbial population during the 5th and the 6th day of the sampling period in treatments T1, T2 and T4 on average were of 19.23x105CFU/ml, in contrast to the population of cellulolytic bacteria in T3, whose recount mean was lower in relation to the other treatments (1.5x105CFU/m lon average) on the 6th day of measurement. When the evaluation of the population growth during the 7th day of the sampling period was made, a higher (p=0.0001) population of viable cellulolytic bacteria was found in T2, in contrast to T1 (good-quality grass content) and T4 (mixture of low quality grass, biomass and cassava flour).
Considering cellulolytic bacteria population in T3, growth in the population was evident, but there were not statistically significant differences in comparison with the other treatments. A statistically significant decrease (p=0.003) in the population was evident, in contrast to treatments T1 and T4 on the 7th day of measurement (Figure 1). Statistically significant differences on the days of population density measurement were evident when the paired comparison, between the collective average recounts of both viable cellulolytic and B. fibrisolvens bacteria, was made. Statistically significant differences were evident in the population density of both viable cellulolytic and B. fibrisolvens from the 7th day, in contrast to the 5th and 6th days of measurement, when the average recount was lower than the one found on the 7th day. The pH of the ruminal content in the different Rusitec fermenters did not change significatively (p=0.165) during the three sampling days, however, a statistically significant decrease in the pH observed in the Rusitec was evident (~pH 6.8), in relation to the pH value that the ruminal flow initial inoculum presented.
qPCR of B. fibrisolvens. The specificity of the qPCR amplification for the ARNr 16S gene partial sequence was assessed by agarose gel electrophoresis at 1%, where a single 246 pb long band was observed, in accordance to the expected size for the reference strain ATCC #19171. In addition, the fusion curve analysis obtained showed a specific and defined curve for each PCR product, without the dimer formation of the primers. High efficiency was observed in the PCR amplification of the analyzed samples, from 80 ng of genomic DNA, with efficiency values within the range of 1.76 to 2, and high linearity between the results obtained in the slope of each sample (Pearson correlation coefficient, r>0.9). Real-time qPCR intra-assay accuracy reached a high level, with a 4% variation coefficient estimated from the Ct data average in each three-repetition cycle, in all samples from the curve under the same experiment. Likewise, real-time qPCR reproducibility of the assessed samples in three experiments performed on different occasions, showed a 12%variation coefficient. The standard curve generated for the quantification of B. fibrisolvens through real-time qPCR presented a linear relation (98% relation coefficient) with an efficiency of 1.84 for the six points included in the standard curve.
The population density of B. fibrisolvens obtained from qPCR initial inoculum was 4.94x106 cells/ml. The density of B. fibrisolvens, during the three sampling days was 9.19x106 cells/ml (media±ESM). Statistically significant differences were not found in the population density of B. fibrisolvens on the fifth and sixth days of sampling with T1 and T4, where population remained constant, whereas a decrease in the population without statistically significant differences was observed in T2. In T3, the population density increased significantly (p=0.045) within a range of 5.2x104 a 1.25x106 cells/ml. However, when the analysis of the population density of B. fibrisolvens during the 7th day was carried out, a statistically significant increase was observable (p=0.001) in T2 (1.18x106 cells/ml). The supplementation of kikuyu grass (19.4% protein) with 20% biomass and 10% cassava flour caused a significant surge in the population of B. fibrisolvens in contrast to the other treatments (Figure 2).
The concentration of vaccenic acid obtained from the initial inoculum was 15 mg/L. Statistically significant differences in the concentration of vaccenic acid in the ruminal content from the Rusitec, during the sampling period, for treatments T1, T3 and T4, were not found. However, in T2, there was a statistically significant increase (p=0.001) in the concentration of this acid. Also, a positive correlation between the amount of cells/ml of B. fibrisolvens and the concentration of vaccenic acid, was evident (r=86%, p=0.0038). In other words, the vaccenic acid concentration was directly proportional to the population density of B. fibrisolvens in the ruminal content during the Rusitec simulation.
DISCUSSION
In this study, it was observed that population density of cellulolytic bacteria and specifically B. fibrisolvens, increased significantly in response to the supplementation of kikuyu grass (19.4% protein) with cassava flour and biomass effluent from ethanol production. It may be due to the starch and protein input in this treatment, contrary to other treatment where the percentage of starch and protein were lower (13) which may have stimulated the amilolytic and proteolytic activity of this type of bacteria (4,11). Likewise, research carried out by Wanapat et al (13) reports that cassava chip and other forms of cassava root can be successfully fermented with yeast (Saccharomyces cereviceae) to obtain a final product with high Crude protein and a relatively high profile of amino acids. It was found that cassava could replace soybean meal completely and was beneficial to cattle in terms of the efficiency of rumen fermentation, microbial protein synthesis, nitrogen retention and nutrient digestibility. Nevertheless, the results from this study in treatments T3 and T4 were completely opposite to results in T2, which may suggest that the positive response to supplementation in T2, relies on the nutritional quality of kikuyu grass.
Studies carried out by Garcia et al (20) assessed the effect of supplementation with biomass obtained as a byproduct from ethanol production by replacing different percentages of protein, of contrasting nutritional quality (high, mid, low) in three kikuyu grass fodders, with protein from biomass. After the evaluation of the fractions (dry matter, neutral detergent fiber and acid detergent fiber) researchers observed that supplementation had a significant positive effect (p<0.01) on the fermentation parameters evaluated with the high-quality grass, in contrast to the results obtained with the supplementation of mid and low-quality kikuyu grass. This suggests that the positive response to supplementation with biomass is related with the nutritional quality of kikuyu grass, which may also influence the population of cellulolytic and B. fibrisolvens bacteria observed in this study in T2, which included the supplementation of high-quality grass with biomass and cassava flour.
The genus Butirivibrio may be divided in several groups corresponding to three different biotypes, in accordance to its capacity to produce some fatty acids. Such fact classifies them as lactic and succinic acid producers, instead of acetate and butyrate producers (6, 21, 22). Mrazek et al (23), identified that the PseudoButyrivibrio ruminis and Butyrivibrio proteoclasticum isolations found in bovine rumen, produce succinate and propanoate, but they do not produce butyric and acetate, in contrast to the type strain for B. fibrisolvens, which produces butyric and lactic acids and formic and succinic acids at a lower quantity. The species of this genus belong to the cluster XIVa of the Subphylum Clostridium and take part in important functions of the ruminal ecosystem, including proteolysis process for fiber degradation and fatty acids biohydrogenation (13,21,24). They also have the ability to hydrogenate linoleic to vaccenic acid, through enzymatic activity, linoleic acid isomerase and linoleic acid reductase, conferring specificity to the use of linoleic acid for conjugated linoleic acid formation (6). Previous research has showed that the synthesis and concentration of CLA in milk and meat from bovines may be enhanced or inhibited by factors such as the microbial composition of the rumen, animal breed, bromatologic composition of pastures and supplementation with other food rich in protein, lipids and fiber and even with some geographical conditions, such as altitude (3,12,13).
In this study, a significant increase in the vaccenic acid concentration was detected in T2 in Rusitec, this is a monounsaturated acid trans that predominates in the fat fraction of the products of ruminal origin and it originates as intermediate product, during the microbial biohydrogenation of unsaturated fatty acids (22). Its concentration is directly related with the diet supplied to animals, which is rich in linoleic, alphalinoleic acid, protein and fiber and requires active participation of the ruminal microorganisms in charge of such process, as it is the case for ruminal bacteria B. fibrisolvens (6,7,13,24). Although during this study a direct quantification of CLA production was not carried out, due to the use of a ruminal simulation system in vitro, instead of meat and dairy products, it was possible to observe that the vaccenic acid concentration in Rusitec was directly proportional to the population density of B. fibrisolvens under the supplementation of kikuyu grass with cassava flour and biomass. These results suggest that the application of this type of supplementation in the field may cause a significant increase in the CLA concentration in milk and meat from bovines, if the role of vaccenic acid as one of its main originators is considered (6,24).
In a parallel study to this research, it was observed that the supplementation of kikuyu grass with cassava flour and biomass (T2) had an statistically significant effect on some digestibility indicators, such as the degradation of dry and organic matter and the production of ammonia and did not have any significant effect on the degradation of the cell wall, raw protein and gas production (13,20). The results from this study, regarding the significant increase of viable cellulolytic and B. fibrisolvens bacteria population density, as well as the vaccenic acid concentration in response to the supplementation of kikuyu grass with cassava flour and biomass, together with the results obtained by García et al (20), in regard of the ruminal digestibility indicators, suggest that this type of diet could enhance significatively the metabolic capacity of ruminal microorganisms and, consequently improve the energetic balance of bovines, which would result in an increase of the nutritional quality of products derived from cattle, as it is the case of milk and meat (6). It has been reported that cassava is a basic energetic food in the industry of balanced food for animals without any relevance to it being flour, flake or granular type (13). Cassava is a highly energetic compound, due to its content of dry matter, cellulose, hemicellulose, protein, starch and other polysaccharides easily fermentable (9,13). Likewise, biomass (protein-rich yeast) effluent from ethanol production, also presents high content of dry matter, protein, lignin, cellulose and hemicelluloses (9, 13).
In conlusions the results from this study, although gathered from an in vitro system, suggest the nutritional potential of cassava flour and biomass effluent from ethanol production, as supplements in grazing bovines feeding. However, additional field research is required to assess the effect of supplementation with cassava flour and biomass in the diet for bovines, and to analyze important indicators such as diet palatability, weight gain, milk production, as well as the in situ effect on the population of ruminal microorganisms, including the characterization of cellulolytic and B. fibrisolvens bacteria, that together, may be useful to determine whether these additives can be used in the formulation of diets for cattle.
Competing interests
The authors declare that they have no competing interests.
Acknowledgements
We thank funding from Ministerio de Agricultura y Desarrollo rural de Colombia (MADR-code-2008D31067-3724) and CODI-Menor cuantía 2010 of Universidad de Antioquia (UdeA). CEAGRO of Universidad Nacional de Colombia sede Medellin (UNALMED), Departamento de Formación Académica de Haciendas-UdeA and the administrative and technical staff from La Montaña farm-UdeA and Paysandú farm-UNALMED.
REFERENCES
1. Barahona R, Sanchez S. Physical and chemical limitations to the digestibility of tropical forages and strategies to overcome them. Revista CORPOICA 2005; 6(1):69-82.
2. Denman SE, McSweeney CS. Development of a real-time PCR assay for monitoring anaerobic fungal and cellulolytic bacterial populations within the rumen. FEMS Microbiol Ecol 2006; 58(3):572-582.
3. Hernandez-Sanabria E, Goonewardene LA, Wang Z, Durunna ON, Moore SS, Guan LL. Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl Environ Microbiol 2012; 78(4):1203-1214.
4. Or-Rashid M, Wright T, McBride B. Microbial fatty acid conversion within the rumen and the subsequent utilization of these fatty acids to improve the healthfulness of ruminant food products. Applied Microbiology and Biotechnology. 2009; 84(6):1033-1043. [10.1007/s00253-009-2169-3].
5. Belenguer A, Toral PG, Frutos P, Hervas G. Changes in the rumen bacterial community in response to sunflower oil and fish oil supplements in the diet of dairy sheep. J Dairy Sci 2010; 93(7):3275-3286.
6. Kaleem M, Enjalbert F, Farizon Y, Troegeler-Meynadier A. Effect of chemical form, heating, and oxidation products of linoleic acid on rumen bacterial population and activities of biohydrogenating enzymes. J Dairy Sci 2013; 96(11):7167-180.
7. Toral PG, Shingfield KJ, Hervás G, Toivonen V, Frutos P. Effect of fish oil and sunflower oil on rumen fermentation characteristics and fatty acid composition of digesta in ewes fed a high concentrate diet. J Dairy Sci 2010; 93:4804–4817.
8. Vergara -Lopez J, Araujo-Febres O. Producción, Composición Química y Degradabilidad Ruminal In Situ de Brachiaria humidicola (RENDLE) Schweick en el Bosque Seco Tropical. Rev Cient (Maracaibo). 2006; 16(3):239-248.
9. Arroyo H, Guardia M, Flórez J. Caracterización bromatológica de materias primas y subproductos en el minucipio de Quibdó, Chocó. Revista Institucional Universitaria tecnológica del Chocó 2007; 26(2):9-12.
10. Singh KM, Tripathi AK, Pandya PR, Parnerkar S, Rank DN, Kothari RK, et al. Use of real-time PCR technique in determination of major fibrolytic and non fibrolytic bacteria present in Indian Surti buffaloes (Bubalus bubalis). Pol J Microbiol 2013; 62(2):195-200.
11. Wanapat M. Potential uses of local feed resources for ruminants. Trop Anim Health Prod 2009; 41(7):1035-1049.
12. McKain N, Shingfield KJ, Wallace RJ. Metabolism of conjugated linoleic acids and 18 : 1 fatty acids by ruminal bacteria: products and mechanisms. Microbiology 2010; 156(2):579-588.
13. Wanapat M, Kang S, Polyorach S. Development of feeding systems and strategies of supplementation to enhance rumen fermentation and ruminant production in the tropics. J Anim Sci Biotechnol 2013; 4(1):32.
14. Correa CHJ, Pabón RML, Carulla FJE. Valor nutricional del pasto kikuyo (Pennisetum clandestinum Hoechst Ex Chiov.) para la producción de leche en Colombia (Una revisión): I - Composición química y digestibilidad ruminal y posruminal. Livestock Research for Rural Development 2008; 20(4). http://www.lrrd.org/lrrd20/4/corra20059.htm
15. Ríos J, Gallego A, Vélez L, Agudelo J, Toro L, Lema A, et al. Caracterizacion y evaluacion de agroecositemas a escala predial. Un estudio de caso; Centro agropecuario Paysandú (Medellin, Colombia). Revista Facultad Nacional de Agronomía 2004; 57( 2).
16. Martinez ME, Ranilla MJ, Tejido ML, Ramos S, Carro MD. Comparison of fermentation of diets of variable composition and microbial populations in the rumen of sheep and Rusitec fermenters. I. Digestibility, fermentation parameters, and microbial growth. J Dairy Sci 2010; 93(8):3684-3698.
17. Czerkawski JW, Breckenridge G. Design and development of a long-term rumen simulation technique (Rusitec). Br J Nutr 1977; 38(3):371-384.
18. Londoño FA, Fernández CJ, Molina GL, Polanco ED, Gutiérrez BL. Cuantificación de bacterias celulolíticas anaerobias provenientes del rumen de ganado bovino: comparación de tres técnicas. Revista Hechos Microbiológicos. 2011; 2(1):51-59.
19. Grubb JA, Dehority BA. Variation in colony counts of total viable anaerobic rumen bacteria as influenced by media and cultural methods. Appl Environ Microbiol 1976; 31(2):262-267.
20. García W, Giraldo L A. Composición química del destilado de yuca y su efecto sobre los parámetros de fermentación ruminal del pasto kikuyo in vitro. Livestock Research for Rural Development [en línea ]. 2014 Diciembre 18; 26: URL disponible en: http://www.lrrd.org/lrrd26/5/garc26086.html.
21. Paillard D, McKain N, Chaudhary LC, Walker ND, Pizette F, Koppova I, et al. Relation between phylogenetic position, lipid metabolism and butyrate production by different Butyrivibrio-like bacteria from the rumen. Antonie van Leeuwenhoek 2007; 91(4):417-422.
22. Wallace RJ, McKain N, Shingfield KJ, Devillard E. Isomers of conjugated linoleic acids are synthesized via different mechanisms in ruminal digesta and bacteria. J Lipid Res 2007; 48(10):2247-2254.
23. Mrazek J, Tepsic K, Avgustin G, Kopecny J. Diet-dependent shifts in ruminal butyrate-producing bacteria. Folia Microbiol (Praha) 2006; 51(4):294-298.
24. Zhao XH, Liu CJ, Liu Y, Li CY, Yao JH. Effects of replacing dietary starch with neutral detergent-soluble fibre on ruminal fermentation, microbial synthesis and populations of ruminal cellulolytic bacteria using the rumen simulation technique (RUSITEC). J Anim Physiol Anim Nutr (Berl) 2012; 97(6):1161-1169.