Single dose secnidazole treatment efficiency against naturally occuring Giardia duodenalis infection in calves
Eficacia del tratamiento con secnidazol en dosis única contra la infección natural de Giardia duodenalis en terneros
Single dose secnidazole treatment efficiency against naturally occuring Giardia duodenalis infection in calves
Revista MVZ Córdoba, vol. 23, no. 2, 2018
Universidad de Córdoba
Received: 06 February 2017
Accepted: 05 June 2017
Abstract: Objective. In the present study the aim was to establish the efficacy of 30 mg/kg single dose secnidazol in calves naturally infected with Giardia duodenalis. Materials and methods. In an attempt to perform original study a total of 18 calves, from various breed, age and of both sexes were enrolled. Diagnosis was based on detection of trophozoit and/or cysts on fecal flotation among calves naturally infected with G. duodenalis, besides by use of rapid diagnostic test kits working with solid phase immunochromatographic principles and β-giardin nested- Polymerase Chain Reaction aplication. Cyst count per gram of feces were performed among days 0, 3, 7 and 10 in all cases. On days 0 and 10 hematological (WBC, RBC, HCT, MCHC, PLT) and serum biochemical (ALT, AST, creatinine, urea) values were determined. Results. Two different groups of calves composed of secnidazole group (n:9) and control group (n:9) were enrolled. Among calves enrolled in treatment group secnidazole was administered at a single dosage of 30 mg/kg perorally on day 0, whereas control group were left without any active ingredient. Cyst count per gram feces, hematological and serum biochemical values were analyzed among groups and intragroup comparisons. Giardia duodenalis assemblage A3 was detected in all 18 calves. On days 3, 7, and 10 there was significant (p˂0.001) reduction in cyst excretion; whereas evaluation of mean geometric cyst excretion revealed 100% reduction on days 7 and 10. Among two group statistical analysis of hematological and serumbiochemical variables revealed no statistical significance on days 0 and 10. Conclusions. In conclusion secnidazole at a single dose of 30 mg/kg might be practically appliable, reasonably priced, safety, completely effective and causing rapid recovery treatment protocole for therapy of calves with Giardiasis.
Keywords: Giardia duodenalis, calf, β-giardin nested PCR, secnidazol, treatment.
Resumen: Objetivo. En el presente estudio se pretendía establecer la eficacia de 30 mg / kg de dosis única de secnidazol en terneros naturalmente infectados con Giardia duodenalis. Materiales y métodos. En un intento de realizar un estudio original, se matricularon 18 terneros, de distintas raza, edad y de ambos sexos. El diagnóstico se basó en la identificación de trofozoítos y /o quistes en la flotación fecal entre terneros naturalmente infectados con G. duodenalis, además de utilizar kits de diagnóstico rápido que funcionan con principios inmunocromatográficos en fase sólida y aplicación de Reacción en Cadena de Polimerasa anidada con β-giardina. El conteo de quistes por gramo de heces se realizó entre los días 0, 3, 7 y 10 en todos los casos. En los días 0 y 10 se determinaron los valores hematológicos (WBC, RBC, HCT, MCHC, PLT) y suero bioquímico (ALT, AST, creatinina, urea). Resultados. Se inscribieron dos grupos diferentes de terneros tratados con secnidazol (n: 9) y el grupo control (n: 9). Entre los becerros incluidos en el grupo de tratamiento se administró secnidazol en una dosis única de 30 mg/kg oralmente al día 0, mientras que el grupo de control se dejó sin ningún ingrediente activo. Se analizó el conteo de quistes por gramo de heces, valores hematológicos y bioquímicos de suero entre grupos y comparaciones intragrupo. Giardia duodenalis ensamblaje A3 se identificó en los 18 terneros. En los días 3, 7 y 10 hubo una reducción significativa (p˂0.001) en la excreción de quistes; Mientras que la evaluación de la excreción geométrica promedio de quistes reveló una reducción del 100% en los días 7 y 10. Entre los dos grupos de análisis estadístico de las variables hematológicas y sero-bioquímicas no reveló significación estadística en los días 0 y 10. Conclusión. En conclusión secnidazol en una dosis única de 30 mg/kg podría ser prácticamente aplicable, a un precio razonable, la seguridad, completamente eficaz y causando un tratamiento de recuperación rápida protocolo para la terapia de terneros con Giardiasis.
Palabras clave: Giardia duodenalis, calf, β-giardin nested PCR, secnidazol, tratamiento.
INTRODUCTION
The protozoan Giardia duodenalis (syn. Giardia intestinalis, Giardia lamblia) has emerged as a significant parasite of livestock (1). Several previous reports indicated giardiasis in calves with changing prevalences and molecular analysis (2,3,4,5,6,7,8,9,10,11). Zoonotic Giardia duodenalis assemblage subgenotype A has been reported in up to 20% of either pre-weaned calves and post-weaned calves (12,13,14,15).
Even though livestock have emerged as a significant host for giardiasis, relatively few researches analyzed the efficacy of treatment in calves. Traditional anti-giardial armamentarium is composed of nitroimidazole compounds as first choice for many years (16,17) on the field and researches in veterinary Medicine. Given the latter compounds partial efficacy, cases refractory to therapy applications are widely observed (18). Given metronidazole as the most significant member of the nitroimidazole class available for therapy of giardiasis, the U.S. Food and Drug Administration has never approved it and resistance to metronidazole has been induced in vitro (19). It should not be unwise to draw suggestion that there is a necessity for establishing a new therapy choice in small ruminants. Thus in the present study, the purpose was to detect the efficacy of 30 mg/kg single dose secnidazol in calves naturally infected with Giardia duodenalis.
MATERİALS AND METHODS
Study design and calves population. The present field study was enrolled among 18 calves from two different farms, to those of special dairy farming operated as single/individual enterprise located in Aydın. The calves had a history of acute diarrheoa, of 2 different breeds (4 Simmental and other 14 Holstein calves) of both sexes, at 20 to 75 days of age. The study protocol was approved by the institutional laboratory animals ethics committee of Adnan Menderes University HADYEK (with no:64583101/2014/119 and date 29/08/2014). Informed written consent was obtained from owners resided on the farms prior to initiation of the study. Necessary ethical guidelines were taken into consideration. Enrolled calves with naturally occuring giardiasis (n=18) were randomly (by coin toss) assigned into 1 of the 2 groups, as follows (Table 1).
| Control group (n=9) | placebo |
| Treatment group (n=9) | Secnidazole* (30 mg/kg, Flagentyl® 500 mg tblt., Eczacıbasi, Turkey) |
Both group of calves, were housed in seperate boxes for preventing cross-contamination during the study. At the beginning of the study on day 0, the individual boxes where the calves were kept, were disinfected with a quaternary ammonium product. All calves were clinically examined within necessary laboratory analysis. All calves initially were observed for the presence of giardiasis, by ZnSO4 flotation. Due to ethical concerns, only 9 calves were served as controls. Following completion of the study, all positive control calves were also treated with secnidazol.
Laboratory analysis
Haematological and serum biochemical analysis. Among aforementioned on days 0 and 10 hematological [(White blood cell (WBC), red blood cell (RBC), packed cell volume (HCT), mean corpuscular hemoglobin concentration (MCHC), platelet (PLT)] and serum biochemical (ALT, AST, creatinine, urea) values were determined in an attempt to diagnose the hematological alterations during giardiasis by use of Abacus junior Vet 5 hematology cell counter and Samsung biochemistry analyzer, respectively. Fot his purpose a total 10 ml of blood was withdrawn from jugular vein from each calf enrolled and immediately forwarded to the lab.
Fecal Examination. During the allocation period (10 days) all calves were screened on days 3,7, and 10. in an attempt to screen the presence/absence of Giardia sp. cysts. Initial day, before any drug administration, was designated as day 0 (D0), whereas D10 was denoted as the end of study. In each calf at least 2 native smear was used, in which fecal material was throughly mixed WITH 33% ZnSO4 solution then were transposed on to centrifuge tubes. Consequently, spinning in centrifuge [at 880 x g for 300 seconds] was achieved. Afterwards, selected and small portion of the fecal mixture was obtained, which then were transposed on a microscope slide covered with Lugol iodine. Achieved slide was observed under microscope, 40x power for probable determination of Giardia cysts. This was repeated twice for several samples obtained on D0. To those of solely mono infected calves with giardiasis were proven solely by microscopical examination. In addition Giardia antigen in calf feces were also analyzed with rapid immunochromatographic test kits for detection of giardiasis (Bovid-5 ® Anigen, Korea, solid phase immunochromatographic assay for rapid diagnosis of antigens directed against Giardia sp., Cryptosporidium sp., Rotavirus, Coronavirus and Escherichia coli k99.
Assessment of treatment efficacy. Secnidazol treatment efficacy in calves involved in this research was assessed by microscopic examination of fecal samples collected on D0 and D10, for prevention of bias that would interfere due to probable reinfection, and measured based on the reduction in cyst output in 2 gropus of calves. Calculation of the reduction in cyst excretion was performed by use of the Henderson–Tilton formula, composed of geometric mean cyst counts similar to those of described previously by Geurden et al (21) and adapted by previous studies (22,23,24).
Molecular analysis. Among 18 calves involved in this research, DNA was extracted throughly from fecal material by use of QIAamp DNA Stool Mini Kit (Qiagen, Germany) taking into account the manufacturer’s manual. Polymerase chain reaction (PCR) analysis were occupied to genotype similar to what has been described previously by Lalle et al (25). Molecular analysis was performed via PCR amplification and sequencing of the 511 bp region of the β-giardin gene.
Statistical analysis. Statistical analysis comprised SPSS statistical software package with version 15; SPSS Inc., Chicago, IL. Faecal cyst count and related data in secnidazol treatment and control groups were analyzed for normality by use of Kolmogorov–Smirnov test. Even if faecal cyst count was not normally distributed; therefore, the data were log-transformed to achieve near-normality. Wilcoxon test was used in an attempt to comperatively evlaute faecal cyst counts before (D0) and after secnidazol treatment (D10). Intragroup baseline cyst value was compared via Mann-Whitney U test. Probability (p<0.05) was deemed significant. Total data were shown as geometric mean and range.
RESULTS
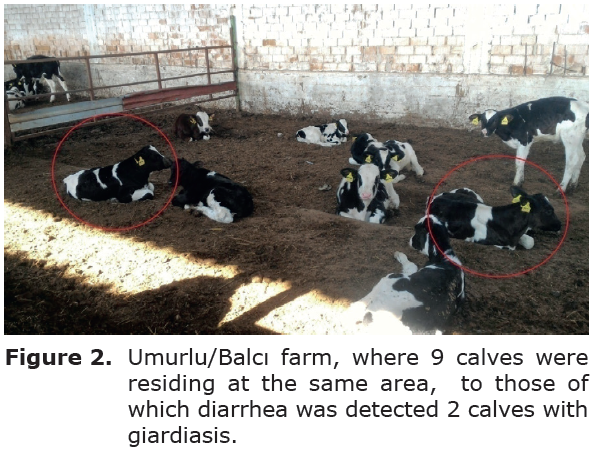
Clinical findings. A total of 18 calves were enrolled (n=4 Simmental and n=14 Holstein calves). Calves were randomly classified into the groups.The geographical locations of the enterprises and demographic data regarding photos of the cases to those of which were included and shown on Google map. (Figure 1). All small scaled farms (Figure 2) were included were loctaed in Aydin municipality of Turkey with geographic data: latitude: 37.8502777778 , longitude: 27.8416666667 0. Clinical signs of diarrhoea were observed among all calves as shown in figure 3.

Haematological and serum biochemical analysis. Among aformentioned parameters there was no intragroup or intergroup differences, as detected by mean values and statistical analysis, which were shown in tables 2 and 3.
| Grup | Working period | Wilcoxon test | Mann-Whitney U test | ||
| Day 0 | Day 10 | P value | P value (Day 0) | P value (Day 10) | |
| Mean WBC (109/l) | |||||
| Treatment | 8.3889 | 7.0522 | 0.214 | 0.546 | 0.863 |
| Control | 7.4756 | 8.2789 | 0.314 | ||
| Mean RBC (1012/l) | |||||
| Treatment | 7.5944 | 6.8033 | 0.314 | 0.136 | 0.863 |
| Control | 6.8711 | 6.9489 | 0.594 | ||
| Mean HGB (g/dl) | |||||
| Treatment | 9.0444 | 8.4000 | 0.514 | 0.489 | 0.63 |
| Control | 9.6667 | 10.0333 | 0.483 | ||
| Mean HCT (%) | |||||
| Treatment | 29.16 | 25.96 | 0.314 | 0.97 | 0.08 |
| Control | 31.50 | 30.45 | 0.678 | ||
| Mean MCV (fl) | |||||
| Treatment | 38.00 | 37.00 | 0.677 | 0.796 | 0.258 |
| Control | 37.66 | 38.97 | 0.722 | ||
| Mean MCHC (pg) | |||||
| Treatment | 32.22 | 29.13 | 0.208 | 0.387 | 0.387 |
| Control | 31.48 | 31.60 | 0.767 | ||
| Mean PLT (109/l) | |||||
| Treatment | 272.88 | 361.77 | 0.021 | 0.436 | 0.024 |
| Control | 323.77 | 289.88 | 0.374 | ||
| WBC: leukocyte, RBC: erythrocytes, HCT: hematocrit, HGB: hemoglobin, MCV: mean erythrocyte volume, MCHC: mean erythrocyte hemoglobin concentration, PLT: thrombocyte N: number of animals | |||||
| Grup | Working period | Wilcoxon test | Mann-Whitney U test | |||
| Day 0 | Day 10 | P value | P value (Day 0) | P value (Day 10) | ||
| Mean ALT (U/l) | ||||||
| Treatment | 18.66 | 18.78 | 0.86 | 0.94 | 0.34 | |
| Control | 22.00 | 41.00 | 0.95 | |||
| Mean AST (U/l) | ||||||
| Treatment | 39.33 | 35.55 | 0.59 | 0.86 | 0.39 | |
| Control | 39.66 | 41.00 | 0.34 | |||
| Mean Creatinine (mg/dl) | ||||||
| Treatment | 0.86 | 0,8933 | 0.44 | 0.73 | 0.79 | |
| Control | 0.86 | 0,8644 | 0.91 | |||
| Mean Urea (mg/dl) | ||||||
| Treatment | 19.33 | 20.66 | 0.44 | 0.07 | 0.09 | |
| Control | 21.44 | 22.55 | 0.35 | |||
| ALT: Alanine aminotransferase, AST: Aspartate aminotransferase | ||||||
Fecal Analysis.
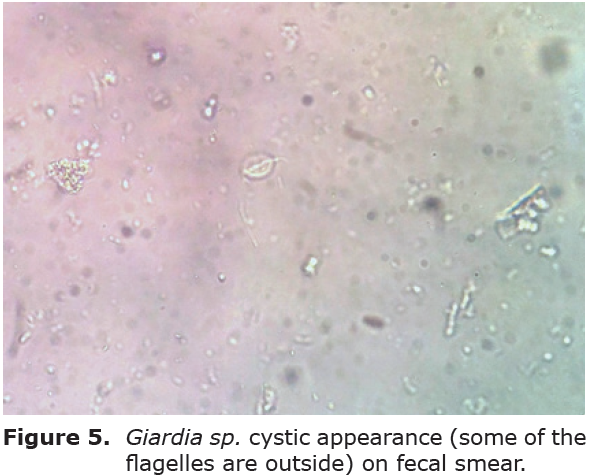
Cyst excretion. Among 18 calves enrolled 7 of them presented cysts among fecal microscopic examination (Figure 4 and 5). Cyst counts and related results of the were presented in Table 3. Throughout the study period calves in control group remained positive with a significant cyst output, showing alteration on day 10 (ranged between 4000 to 140000) compared to the basline values (ranged 8000-48000), whereas there was no statistical significance. Contrarily treatment group calves showed cyst counts ranged between 8000-280000 on D0, whereas fecal samples without any detectable cysts of Giardia sp. included samples from all calves on days 7 and 10 (Table 3). On days 7 and 10, after secnidazol treatment, the percentage reduction in cyst excretion calculated based on geometric mean was 100%. In treatment group geometric mean for cyst excretion was significantly decreased (p<0.001) after treatment. Between groups there were significant differences on days days 3, 7, and 10 (p<0.01)(Table 4) .

| Day 0 XG | (min-max) | Day 3 XG | (min-max) | Day 7 XG | (min-max) | Day 10 XG | (min-max) | |
| Control group | 15979.93a | 8000-48000 | 14035.64 a | 4000-256000 | 12924.98 a | 4000-80000 | 20592.60 a | 4000-140000 |
| Treatment group | 29063.92 a | 8000-280000 | 6.31 b | 0-4000 | 0 b | 0-0 | 0 b | 0-0 |
| P value | 0.258 | 0.000 | 0.000 | 0.000 | ||||
| Decrease in cyst excretion (%) | %99.97 | %100 | %100 | |||||
| a, b: The differences between the averages with different letters in the same row and column are important. (P˂0.001) (P˂0.05) P: Statistical comparison of treatment and treatment groups | ||||||||
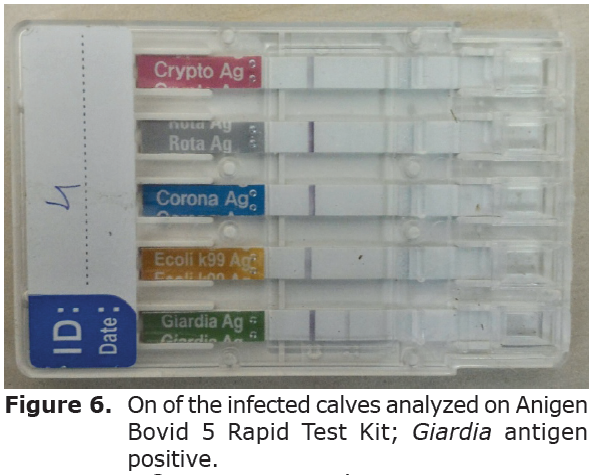
Immunochromatographic test results. All calves were tested by Bovid-5 test kits showed positivity against G. doudenalis (Figure 6). To those of only mono-infected calves were chosen.
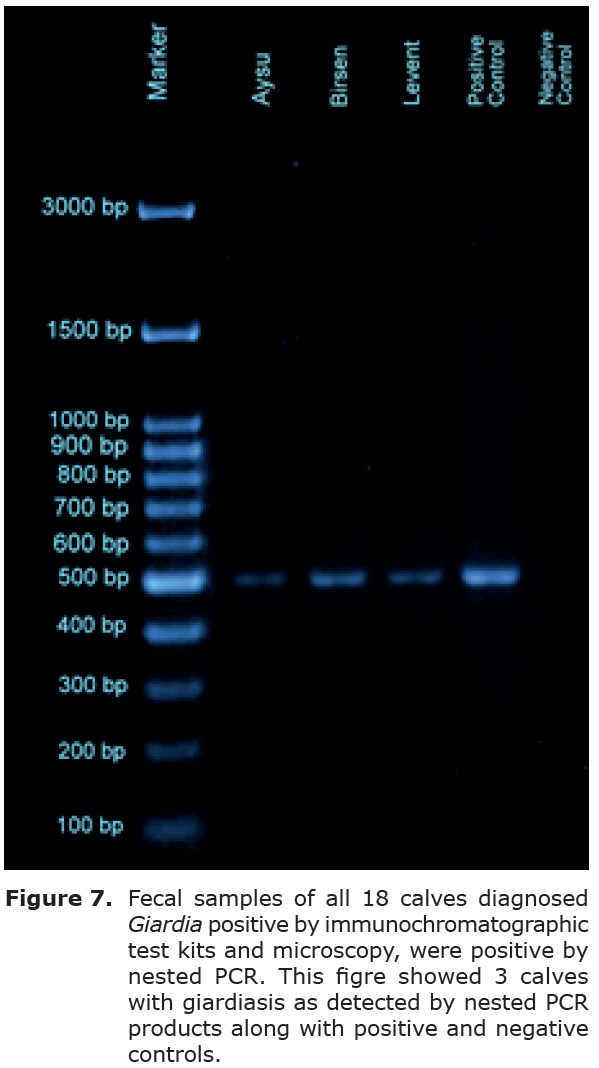
PCR amplification and sequence analysis of the β -giardin gene. Fecal samples of all 18 calves diagnosed Giardia positive by immunochromatographic test kits and microscopy, were positive by nested PCR (Figure 7). PCR products from each of the 18 Giardia-positive calf fecal samples were forwarded to molecular characterization. The β-giardin nested PCR assay was successfully applied to 18 isolates. The achieved DNA sequence from the selected isolates was compared with reference sequences (GenBank ® accession number:M36728 for sub-assemblage A1, AY072723 for sub-assemblage A2, AY072724 for sub-assemblage A3). A3 subgenotype (13,15,26) of assemblage A was isolated from all 18 calves enrolled.
DISCUSSION
Limited literature and treatment options are currently available for combatting against giardiasis in calves. Chloroquine (24,26) as a novel therapeutic choice has found great attention in the literature, in which must be investigated in larger calf populations. On the other hand previous literature indicating the efficacy of secnidazol against giardiasis in sheep (22,23), mice (27) all gave positive and encouraging results. As there was no previous study investigated secnidazol efficacy against giardiasis in calves, the proposed dosage in this study was adapted from previous works which included sheep (22,23) with giardiasis and cattle with balantidiasis (30).
Secnidazol has been the subject of prior studies regarding antigiardial treatment in sheep (25,29), cats (30) and mice (29). To the present authors’ knowledge complete (100%) efficacy (on days 7 and 10 following treatment) of a single dose of an oral treatment with secnidazole at proposed dosage for combatting against natural giardiasis in calves has been detected for the first time. In the present field and clinical trial, secnidazole caused no side effects nor clinical signs, thus did not require treatment intervention. Available evidence in this study suggested that secnidazole might be efficacious as other 5-nitroimidazole drugs as a therapeutic armamentarium for giardiasis in calves.
In a prior multidiciplinary research at agricultural and veterinary fields the efficacy of secnidazol treatment on milk production in dairy ewes infected with G. duodenalis was assessed. Single secnidazole at a dose of 10 mg/kg-30 mg/kg, revealed that there was a significant reduction in cyst excretion (22). Another study determined secnidazole administration at a single dose of 10 mg/kg, orally in lambs naturally infected with G. duodenalis. Twelve weeks of age weaned lambs were selected and randomly assigned into two groups based on placebo (n=7 untreated control group) or treatment (n=10 lambs treated with a single secnidazole; dosage 10 mg/kg). During the latter study a high (99.98%) reduction in cyst excretion in the secnidazol treatment group compared to the control group at day 10, revealed a significant (p<0.001) reduction in cyst excretion (23). Another study reported the efficacy of three treatments measured against Balantidium coli, which is another parasite, in cattle. According to the latter authors obtained therapeutic efficacy rates as 37.5, 62.5, and 87.5% for metronidazole, oxytetracycline, and secnidazole, making secnidazole the most effective option (28).
In the present study Secnidazole treatment efficacy in calves might be comperatively discussed to what has been described in lambs naturally infected with giardiasis, in which a single and oral dosage of 30 mg/kg caused 99.98% efficacy, with no more cyst was determined in faeces 10 days after treatment (23). In the present study secnidazole therapy entirely and significantly reduced the cyst excretion calculated based on geometric mean by 100% on day 7 and 10 after the initiation of the treatment, resulting in a significant reduction (p<0.001) in cyst excretion. It may be briefly suggested that the proposed single dosage of the latter compound, ease of administration, well toleration of calves, all could contribute making it a superior therapeutic armamentarium in contrast to other relevant nitroimidazole compounds
Given the efficacy of secnidazole (as it resulted in a significantly decreased cyst excretion) as a single dose for treatment of naturally occuring Giardia infection among Sakiz lambs involved in the present study, the usage of this of drug should be encouraged. The high cyst reducing activity of secnidazol against G. duodenalis may provide an important benefit in a clinical setting where a specific diagnosis is impossible or impractical, likewise livestock field condition. The availability of this inexpensive drug, the cost (approximately 2.2 dollars per a lamb for therapy) and the easily availability for market opportunity have been significant impediments for usage of this antiparasitic drug against giardiasis in sheep.
Acknowledgements
This study was summarized paritally from a national Project and was funded by Adnan Menderes University Research Projects Funding Unit (ADU-BAP) with project number VTF-15048. This article was a PhD study with thesis no:440320 (Ulusal Tez Merkezi: National Thesis Center) regarding student Göktuğ Toros under advisory of Dr. Kerem Ural.
REFERENCES
1. O’Handley RM, Cockwill C, Jelinski M, McAllister TA, Olson ME. Effects of repeat fenbendazole treatment in dairy calves with giardiosis on cyst excretion, clinical signs and production. Vet Parasitol. 2000; 89(3):209-218.
2. Barigye R, Dyer NW, Newell T, Khaitsa ML, Trout JM, Santin M, Fayer R. Molecular and immunohistochemical detection of assemblage E, Giardia duodenalis in scouring North Dakota calves Vet Parasitol. 2008; 157:196–202.
3. Huang J, Yue D, Qi M, Wang R, Zhao J, Li J, Shi K, Wang M, Zhang L. Prevalence and molecular characterization of Cryptosporidium spp. and Giardia duodenalis in dairy cattle in Ningxia, northwestern China. BMC Vet Res. 2014; 10(292):1-5.
4. Lee SH, VanBik D, Kim HY, Cho A, Kim JW, Byun JW, Oem JK, Oh SI, Kwak D. Prevalence and molecular characterisation of Giardia duodenalis in calves with diarrhoea. Vet Rec. 2016; 178(25):633.
5. Coklin T, Farber JM, Parrington LJ, Coklin Z, Ross WH, Brent R. DixonTemporal changes in the prevalence and shedding patterns of Giardia duodenalis cysts and Cryptosporidium spp. oocysts in a herd of dairy calves in Ontario. Can Vet J. 2010; 51(8):841–846.
6. Gharieb RMA, El-Ghany AMA. Giardia lamblia in household persons and buffalo calves; prevalence, molecular identification and associated risk factors. Japan J Vet Res. 2016; 64(2):15-22.
7. Hamnes IS, Gjerde BK, Robertson LJ. Prevalence of Giardia and Cryptosporidium in dairy calves in three areas of Norway. Vet Parasitol. 2006; 140:204–216.
8. Suman MSH, Alam MM, Pun SB, Khair A, Ahmed S, Uchida RY. Prevalence of Giardia lamblia infection in children and calves in Bangladesh Bangl J Vet Med. 2011; 9(2):177–182.
9. Trout JM, Santín M, Greiner E, Fayer R. Prevalence and genotypes of Giardia duodenalis in post-weaned dairy calves. Vet Parasitol. 2005; 130(3):177-183.
10. Tiranti K, Larriestra A, Vissio C, Picco N, Alustiza F, Degioanni A, Vivas A. Prevalence of Cryptosporidium spp. and Giardia spp. spatial clustering and patterns of shedding in dairy calves from Córdoba, Argentina. Rev Bras Parasitol Vet. 2011; 20(2):140-147.
11. Winkworth CL, Learmonth JJ, Matthaei CD Townsend CR. Molecular Characterization of Giardia Isolates from Calves and Humans in a Region in Which Dairy Farming Has Recently Intensified. Appl Environ Microbiol. 2008; 74(16):5100-5105.
12. Cacciò SM, Thompson RC, McLauchlin J, Smith HV. Unravelling Cryptosporidium and Giardia epidemiology. Trends Parasitol. 2005; 21:430–437.
13. Geurden T, Geldhof P, Levecke B, Martens C, Berkvens D, Casaert S, Vercruysse J, Claerebout E. Mixed Giardia duodenalis assemblage A and E infections in calves. Int J Parasitol 2008; 38(2): 259–264.
14. Itagaki T, Kikawa M, Terasaki K, Shibahara T, Fukuda K. Molecular characterization of parthenogenic Fasciola sp. in Korea on the basis of DNA sequences of ribosomal ITS1 and mitochondrial NDI gene. J Vet Med Sci. 2005; 67:1115–1118.
15. Santina M, Dargatz D, Fayer R. Prevalence of Giardia duodenalis assemblages in weaned cattle on cow-calf operations in the United States. Vet Parasitol. 2012; 183:231–236.
16. Ali V, Nozaki T. Current therapeutics, their problems, and sulfur-containing-amino-acid metabolism as a novel target against infections by “amitochondriate” protozoan parasites. Clin Microbiol Rev. 2007; 20:164–187.
17. Tejman-Yarden N, Eckmann L. New approaches to the treatment of giardiasis. Curr Opin Infect Dis. 2011; 24(5):451-456.
18. Schwartz E, Lachish T, Meltzer E. Treatment of Giardiasis after Nonresponse to Nitroimidazole. Emerg Infect Dis. 2014; 20(10):1738-1740.
19. Boreham PFL, Phillips RE, Shepherd RW. Altered uptake of metronidazole in vitro by stocks of Giardia intestinalis with different drug sensitivities. Trans R Soc Trop Med Hyg. 1988; 82:104-106.
20. Tangtrongsup S, Scorza V. Update on the Diagnosis and Management of Giardia spp. Infections in Dogs and Cats. Topics in Companion Animal Medicine. 2010; 25(3):155-162.
21. Geurden T, Pohleb H, Sarrea C, Dreesena L, Vercruyssea J, Claerebouta E. The efficacy of a treatment with fenbendazole against an experimental Giardia duodenalis infection in lambs. Small Rum Res. 2011; 96(2–3):211–215.
22. Alιç Ural D, Ayan A, Aysul N, Balιkçι C, Ural K. Secnidazol Treatment to Improve Milk Yield in Sheep with Giardiasis. Atatürk Üniversitesi Vet Bil Derg. 2014; 9(2):74-82.
23. Ural K, Aysul N, Voyvoda H, Ulutas B, Aldemir OS, Eren H. Single dose of secnidazole treatment against naturally occuring Giardia duodenalis infection in Sakiz lambs. Rev MVZ Cordoba. 2014; 19(1):4023-4032.
24. Karademir U, Ural K, Aysul N, Ayan A, Toplu S, Ortlek O, Balikci C, Künyeli A, Erdogan H. The efficacy of chloroquine treatment against naturally occuring Giardia duodenalis infection in lambs. Rev MVZ Córdoba. 2016; 21(2):5328-5335.
25. Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D, Cacciò SM. Genetic heterogeneity at the β-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. Int J Parasitol. 2005; 35(2):207-213.
26. Gultekin M, Ural K, Aysul N, Ayan A, Balikci C, Akyildiz G. The efficacy of chloroquine treatment of Giardia duodenalis infection in calves. Vlaams Diergeneeskundig Tijdschrift. 2016; 85:335-341.
27. da Silva AS, da Silva MK, Oliveira CB, Zanette RA, Monteiro SV. Efficacy of drugs against Giardia muris in mice Mus musculus naturally infected Ciencias Agrárias, Londrina, 2008; 29(1):175-178.
28. Bilal CQ, Khan MS, Avais M, Ijaz M, Khan JA. Prevalence and chemotherapy of Balantidium coli in cattle in the River Ravi region, Lahore (Pakistan). Vet Parasitol 2009; 163(1-2):15-17.
29. Alιç Ural D, Aysul N, Gültekin M. The Efficacy of Oral Administration of Clinoptilolite Against Naturally Occuring Giardiasis in Calves. Kocatepe Vet J. 2016; 9(4):288-293.
30. DaSilva AS, Castro VSP, Tonin AA, Brendler S, Costa MM, Jaques JA, Bertoletti B, Zanette RA, Raiser AG, Mazzanti CM, Lopes STA, Monteiro SG. Secnidazole for the treatment of giardiasis in naturally infected cats. Parasitol Int. 2011; 60(4):429-432.