Serological status of IBR, BVD, leucosis, Leptospira and Neospora caninum in bovine females of the department of Santander, Colombia
Estado serológico a IBR, DVB, Leucosis, Leptospira y Neospora caninum en hembras bovinas del Departamento de Santander, Colombia
Serological status of IBR, BVD, leucosis, Leptospira and Neospora caninum in bovine females of the department of Santander, Colombia
Revista MVZ Córdoba, vol. 23, no. 2, 2018
Universidad de Córdoba
Received: 06 February 2017
Accepted: 05 June 2017
Abstract: Objective. To determine the serological status of bovine females for infectious bovine rhinotracheitis (IBR), bovine viral diarrhea (BVD), leucosis, Leptospira and Neospora caninum in the department of Santander, Colombia. Materials and methods. Convenience sampling was conducted on 460 cattle farms in 23 municipalities of Santander (Colombia). The collected sera were analyzed using different commercial ELISA kits following the manufacturer’s instructions. Results. Seroreactive animals were found for all diseases studied, with general prevalence rates of 21.8% for leucosis, 26.1% for Leptospira, 29.7% for BVD, 48.2% for IBR and 63% for Neospora. Conclusions. The prevalence rates of the diseases analyzed in the department of Santander among the bovine population fluctuate from medium to high and thus require official control measures.
Keywords: Bovine, serology, antibodies, enzyme-linked immunosorbent assay.
Resumen: Objetivo. Conocer el estado serológico de hembras bovinas a IBR, DVB, leucosis, leptospira y Neospora caninum en el departamento de Santander, Colombia. Materiales y métodos. Se realizó un muestreo por conveniencia en 460 fincas ganaderas de 23 municipios de Santander (Colombia), los sueros colectados fueron analizados mediante diferentes kits comerciales de ELISA siguiendo las instrucciones del fabricante. Resultados. En todas las enfermedades estudiadas se encontraron animales seroreactores, correspondiendo la prevalencia general a Leucosis 21.8%, Leptospira 26.1%, DVB 29.7%, IBR 48.2% y Neospora 63%. Conclusiones. En el departamento de Santander las enfermedades analizadas se encuentran en la población bovina con prevalencias que fluctúan entre medio a alto, lo cual requiere medidas de control oficial.
Palabras clave: Bovinos, serología, anticuerpos, Ensayo de Inmunoadsorción Enzimática.
INTRODUCTION
Reproductive infectious diseases represent a serious limitation of bovine production. The etiology of these disease is diverse and comprises a variety of viral, bacterial, protozoan, chlamydial and fungal agents, some of which are zoonotic (1). Brucellosis, neosporosis, leptospirosis, bovine viral diarrhea and infectious bovine rhinotracheitis are the most well-known diseases and cause the greatest economic losses for the livestock industry (2). The main alterations caused by these agents are abortions and infertility. These alterations make diagnosis difficult, as more than 50% of abortions have an indeterminate etiology (3). Regardless of the individual losses caused by each agent, the association between them generates different co-infections that can be subclinical and can increase economic losses (4,5).
In contrast to the prevalence of these agents in some European countries, where they are being eradicated (6,7,8), their prevalence in Colombia continues to be high in some regions, and no short-term strategies are available for effective control measures.
This situation may affect the competitiveness of the livestock sector in the future, since disease-free countries can impose health and commercial barriers to meat and other products originating from countries that are not disease-free.
Colombia has a cattle population of 22.689.420, of which 1.412.313 are located in the department of Santander, which makes this department the sixth largest cattle-producing department (9). Although isolated serological studies have been performed in other regions of cattle importance (10,11,12), no studies have been conducted for the department of Santander.
The objective of this study was to determine the serological status of bovine females against IBR, BVD, leucosis, Leptospira and Neospora caninum in the department of Santander, Colombia.
MATERIALS AND METHODS
Location. The work was conducted in 460 cattle farms of 23 municipalities of Santander. Santander is located in the northeastern region of the country and is composed of 87 municipalities grouped into six provinces: Comunera, García Rovira, Guanentá, Mares, Soto and Vélez.
Location. The work was conducted in 460 cattle farms of 23 municipalities of Santander. Santander is located in the northeastern region of the country and is composed of 87 municipalities grouped into six provinces: Comunera, García Rovira, Guanentá, Mares, Soto and Vélez.
A total of 440 blood samples from 460 farms with no vaccination history were collected during the second half of 2015 and assessed by ELISA. The municipalities included in the study were Piedecuesta, Mesa de los Santos, San Gil, Betulia, Guadalupe, San Vicente de Chucuri, Charalá, Puerto Wilches, Barbosa, Cerrito, Málaga, Suaita, Guapota, Barrancabermeja, Puerto Parra, Simacota, Sabana de Torres, Matanza, Miranda, Mogotes, Rio Negro, Vélez and Cimitarra.
Obtaining blood samples. A total of 5.0 ml of blood was obtained from the coccygeal vein of each animal using an 18-gauge needle and collected in Vacutainer ® tubes without anticoagulant. The samples were duly identified, transported at 4°C to the Clinical Veterinary Laboratory of the Cooperative University of Colombia (UCC) and centrifuged at 2,500 revolutions per minute (rpm) for 10 minutes. The resulting sera was fractioned into 2.0 ml vials and stored at -20°C prior to analysis.
ELISA test. Samples were processed using commercial indirect ELISA kits for the detection of antibodies against bovine viral diarrhea (BVD), infectious bovine rhinotracheitis (IBR), enzootic bovine leukosis and Neospora caninum (Svanova Biotech ® ). Indirect ELISAs use immobilized antigens on microplates and detect antibodies in bovine sera.
For the diagnosis of Leptospira, a double antibody sandwich assay (DAS-ELISA) (Linnodee bovine Leptospira Elisa kit) was used to detect antibodies in response to lipopolysaccharides (LPSs) of Leptospira borgpetersenii serovar Hardjo (hardjp bovis) and Leptospira interrogans serovar Hardjo (lPS) hardjo pratjino. In all tests, the procedures used in the laboratory followed the manufacturer’s instructions.
Ethical aspects. The authors state that respectful treatment was given to the animals during the execution of the project proper and that the blood collections were always performed by professionals in veterinary medicine and animal husbandry with the consent of the owners.
Statistical analysis. The information obtained was recorded in a database in Excel and analyzed using descriptive statistics.
RESULTS
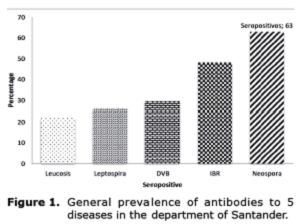
Animals exhibited seroreactivity to all diseases studied, with general prevalence rates as follows: leucosis, 21.8%; Leptospira, 26.1%; BVD, 29.7%; IBR, 48.2%; and Neospora, 63% (Figure 1).
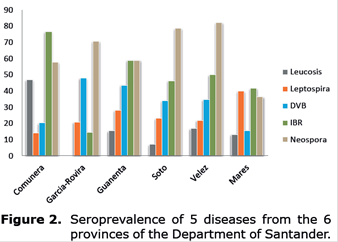
Province-wide, Neospora caninum had the highest seroprevalence, with the highest values in the provinces of Vélez (82.2%), Soto (78.6%), García-Rovira (70.8%), Guanenta (59%), Comunera (57.8%) and Mares %). Regarding IBR, the highest prevalence values corresponded to Comunera (76.6%), Guanenta (59%) and Velez (50%). The highest prevalence of BVD was observed in Garcia-Rovira (47.9%), and the lowest was found in Mares (15.6%), whereas for bovine leucosis, the highest prevalence was detected in Comunera (46.9%), and no reactors were present in Garcia-Rovira (0%). Finally, the highest values for Leptospira were found in Mares (40%), and the lowest values were observed in Comunera (14.1%) (Figure 2).
Regarding the municipalities, the largest numbers of reactors to Neospora caninum were found in Guapota and Simacota and to IBR in Guapota, Mesa de los Santos and Betualia; no reactors were found in Miranda. The distribution of BVD seropositive sera was homogeneous among the municipalities, whereas in some municipalities the prevalence of leucosis was medium to low, and no reactors were present in Cerrito, Malaga, Miranda, Mogotes, Piede Cuesta and Puerto Wilchesin. The lowest values for Leptospira were observed in Sabana de Torres, Suaita, Guapotá, Matanzas and Mesa de los Santos, and no reactors were found in Miranda (Table 1).
| Province | Municipality | Seropositivity | ||||
| Leucosis | Leptospira | BVD | IBR | Neospora | ||
| Comunera (64) | Suaita | 2/9 | 1/9 | 1/9 | 3/9 | 4/9 |
| Guadalupe | 9/14 | 5/14 | 4/14 | 12/14 | 1/14 | |
| Simacota | 9/21 | 2/21 | 4/21 | 15/21 | 16/21 | |
| Guapotá | 10/20 | 1/20 | 4/20 | 19/20 | 16/20 | |
| García-Rovira (48) | Cerrito | 0/20 | 5/20 | 12/20 | 5/20 | 14/20 |
| Málaga | 0/13 | 5/13 | 7/13 | 2/13 | 10/13 | |
| Miranda | 0/15 | 0/15 | 4/15 | 0/15 | 10/15 | |
| Guanenta (39) | San Gil | 1/8 | 2/8 | 3/8 | 6/8 | 2/8 |
| Charalá | 5/14 | 3/14 | 4/14 | 8/14 | 5/14 | |
| Mogotes | 0/17 | 6/17 | 10/17 | 9/17 | 16/17 | |
| Soto (56) | Rio negro | 2/19 | 10/19 | 1/19 | 3/19 | 13/19 |
| Piedecuesta | 0/8 | 1/8 | 6/8 | 8/8 | 7/8 | |
| Matanzas | 1/17 | 1/17 | 4/17 | 4/17 | 12/17 | |
| Mesa de los Santos | 1/12 | 1/12 | 8/12 | 11/12 | 12/12 | |
| Vélez (118) | Cimitarra | 6/16 | 8/16 | 3/16 | 8/16 | 4/16 |
| Vélez | 1/17 | 4/17 | 9/17 | 2/17 | 11/17 | |
| Barbosa | 5/40 | 10/40 | 12/40 | 17/40 | 29/40 | |
| Puerto Parra | 8/45 | 4/45 | 17/45 | 32/45 | 43/45 | |
| Mares (115) | Betulia | 8/13 | 4/13 | 1/13 | 13/13 | 0/13 |
| San Vicente | 1/20 | 9/20 | 4/20 | 3/20 | 2/20 | |
| Barrancabermeja | 22/52 | 22/52 | 9/52 | 25/52 | 25/52 | |
| Sabana de Torres | 5/15 | 2/15 | 1/15 | 5/15 | 5/15 | |
| Puerto Wilches | 0/15 | 9/15 | 3/15 | 2/15 | 10/15 | |
DISCUSSION
The study of reproductive diseases in the main cattle regions of Colombia must be prioritized to establish control plans and enable zonal eradication programs for the improvement of the health status of the country’s herd.
The results of this study show a high presence of antibodies against Neospora caninum (63%) that coincides with studies in several countries (13), indicating that this disease is the major cause of economic losses in dairy and beef cattle worldwide (14). A similar prevalence (57.5%) has been reported in the Sugamuxi Valley (Boyacá) (15), and a higher prevalence has been reported in bulls of Middle Magdalena Valley (79.3%) (16), which is in contrast with the low seropositivity (10.2%) in animals from Montería with reproductive problems (17). The high prevalence in bovine females in this study should be a cause for concern, since this infection has been associated with epidemic abortion in dairy cattle (18), and vertical transmission of the infection can infect the entire herd.
Concomitantly, seropositive animals are more likely (12-19 times) to abort than seronegative animals (19), but this outcome should be contrasted with clinical findings in the field. If this relationship exists, increasing the region’s livestock population in response to the increased demand for meat and milk by domestic and foreign markets will be difficult.
However, this information should be carefully analyzed, since cross-reactions have been found with other cyst-forming parasites of the family Sarcocystidae, including Toxoplasma gondii, Neospora spp., Sarcocystis spp., Hammondia spp. and Besnoitia besnoiti (20), which have not been studied in the country and should be investigated.
The prevalence of IBR (48.2%), which had the second highest prevalence in this study, was similar to that reported in different countries (21). However, within the country, IBR is much more prevalent in other regions, including Antioquia (85.5%), Valle (69.8%) (10), Montería, Córdoba (74.7%) (22), Colombian Altillanura (84%) (23) and Caquetá (90%) (11). In another study, the prevalence in bulls from Middle Magdalena was 92.5% (24). However, bovine herpesvirus 5 (HVB-5), which is a recently detected agent in the country (23), presents a cross-reaction with bovine herpes virus 1 (HVB-1), which is the etiological agent of IBR, and may interfere with these results; this possibility warrants further research. In addition, the latent nature of both herpesviruses suggests that they will continue to circulate indefinitely on farms with seropositive animals with subsequent new infections and reinfections.
Regarding BVD, studies in the country have found a high prevalence in the Bogota savannah (89%) (25) but a progressively lower prevalence in other regions, such as Caquetá (58%) (11), Valle del Cesar (46%) (26) and Montería, Córdoba (29.4%) (12). Notably, this antibody response possibly corresponded to genotype I of the BVD virus, since at the time of the study, genotype 2 did not exist in Colombia (27). The persistence of this virus on the farms studied will depend on the presence of persistently infected (PI) animals, which can affect the entire herd. Thus, further investigation is warranted to detect these individuals.
The prevalence of Leptospira hardjo (26.1%) was lower than the prevalence reported in Central America (31-83%) (28) and other countries in Latin America (16.4-100%) (29). Although establishing comparisons with international and national studies that use microscopic agglutination tests (MAT), which are considered the standard and are based on the use of live antigens, is difficult, the use of ELISA has the advantage of detecting LPS antibodies to the antigenically similar serovar hardjo type hardjo-bovis virus (30).
With this clarification, the results obtained are similar to those found in Montería, Córdoba (20.8%) (31) and Caquetá (28%) (32) but are in contrast to those obtained in the coffee-growing region (45.7%) (33).
With respect to the bovine leukemia virus, which causes enzootic bovine leukosis, the prevalence was lower than the prevalence reported in Peru (42.3%), Paraguay (50%), Bolivia (30%), Argentina (77.4%) and Chile (29.1%) (34) and was similar to that found in the department of Córdoba (21.5%) by ELISA (35). However, the prevalence was high (83% and 60%) in some breeds (Harton del Valle and Chino Santanderano cattle, respectively), which contrasted with the absence of reactors in Blanco Orejinegro, Sanmartinero and Romosinuano cattle and suggested some degree of resistance to the virus (36).
Another study in the Mesa de los Santos (Santander) showed a high prevalence (73%) in dairy cattle (37). In contrast, a lower prevalence (44%) detected by PCR was reported in dairy cattle in the department of Antioquia (38). The use of molecular techniques will likely increase the detection of seropositive animals compared to the use of traditional immunoenzymatic and agglutination techniques, resulting in an increased prevalence in future studies. Although the prevalence found for this virus is not high and economic losses have not been directly associated with reproductive problems, the impact of this virus should not be ignored, since the infected animals have compromised immune systems, which makes them susceptible to suffering from other secondary diseases (39).
The absence of serological reactors to leucosis in the three municipalities of the province of García-Rovira is noteworthy and can be explained by geographic isolation and the absence of roads, which prevent animals from circulating throughout the province. An exception is Cimitarra (Vélez province), where the cattle population is larger and the mobilization of cattle is greater due to a cattle auction that allows greater commercialization of cattle to neighboring farms.
In conclusion, the prevalence of the diseases analyzed in the department of Santander among the bovine population fluctuates from medium to high and thus requires official control measures.
Conflict of interests.
The authors declare no conflict of interest.
REFERENCES
1. Yoo HS. Infectious causes of reproductive disorders in cattle. J Reprod Dev. 2010; 56(Suppl):S53-60.
2. Waldner CL. Serological status for N. caninum, bovine viral diarrhea virus, and infectious bovine rhinotracheitis virus at pregnancy testing and reproductive performance in beef herds. Anim Reprod Sci. 2005; 90:219–242.
3. Jamaluddin AA, Case JT, Hird DW, Blanchard PC, Peauroi JR, Anderson ML. Dairy cattle abortion in California: evaluation of diagnostic laboratory data. J Vet Diagn Invest. 1996; 8(2):210-218.
4. Rinaldi L, Pacelli F, Iovane G, Pagnini U, Veneziano V, Fusco G et al. Survey of Neospora caninum and bovine herpes virus 1 coinfection in cattle. Parasitol Res. 2006; 100 (2): 359-364.
5. Campos FS, Franco AC, Hubner SO, Oliveira MT, Silva AD, Esteves PA et al. High prevalence of co-infections with bovine herpesvirus 1 and 5 found in cattle in southern Brazil. Vet Microbiol. 2009; 139(1-2): 67–73.
6. Ackermann M, Engels M. Pro and contra IBR-eradication. Vet Microbiol. 2006; 113(3-4):293–302.
7. Nuotio L, Neuvonen E, Hyytiäinen M. Epidemiology and eradication of infectious bovine rhinotracheitis/infectious pustular vulvovaginitis (IBR/IPV) virus in Finland. Acta Vet Scan. 2007; 49(3):1-6.
8. Stahl K, Alenius S. BVDV control and eradication in Europe - an update. Jpn J Vet Res. 2012; 60(Suppl):S31–9.
9. Instituto Colombiano Agropecuario, ICA. National Livestock Census. [on line]. ICA: Colombia; 2016. URL available in: http://www.ica.gov.co/getdoc/8232c0e5-be97-42bd-b07b-9cdbfb07fcac/Censos-2008.aspx
10. Ruiz-Sáenz J, Jaime J, Vera VJ. Serological prevalence and isolation of Bovine Herpesvirus 1 (BHV-1) in cattle herds of Antioquia and Valle del Cauca. Rev Colom Cienc Pecua. 2010; 23(3):299-307.
11. Mota-Giraldo JL, Waltero-Garcia I, Abeledo MA. Prevalence of antibodies to Bovine viral diarrhea virus, bovine herpes virus 1, bovine herpes virus 4 in cattle and buffalo from the department of Caquetá. Rev Salud Anim. 2013; 35(3):174-181.
12. Betancur C, Gogorza LM, Martínez FG. Seroepidemiology of Bovine Viral Diarrhea in Monteria (Córdoba, Colombia). Analecta Veterinaria. 2007; 27(2):11-16.
13. Kowalczyk SJ, Czopowicz M, Weber CN, Muller E, Witkowski L, Kaba J. Herd-level seroprevalence of Neospora caninum infection in dairy cattle in central and northeastern Poland. Acta Parasitol. 2016; 61(1):63-65.
14. Bruhn FRP, Daher DO, Lopes E, Barbieri JM, da Rocha CM, Guimarães AM. Factors associated with seroprevalence of Neospora caninum in dairy cattle in southeastern Brazil. Trop Anim Health Prod. 2013; 45(5):1093-1098.
15. Pulido MO, Díaz AM, García DJ, Andrade RJ. Determination of anti-Neospora caninum antibodies in cows from the province of Sugamuxi, Colombia. Rev Mex de Cienc Livestock. 2013; 4(4):501-506.
16. Camacho R, Carvajal LY, Castellanos-Dominguez YZ, Díaz WF, Vásquez MC. Presence of IgG antibodies against reproductive infections in breeding bulls of Magdalena Medio, Colombia. Rev Colom Cienc Pecua. 2015; 28(4):323-330.
17. Serological study on neosporosis in cattle with reproductive problems in Monteria, Córdoba, Colombia. Rev MVZ Córdoba. 2007; 12 (1): 929-933.
18. McAllister MM. Diagnosis and Control of Bovine Neosporosis. Vet Clin North Am Food Anim Pract. 2016; 32(2):443-463.
19. López-Gatius F, López-Béjar M, Murugavel K, Pabón M, Ferrer D, Almeria S. Neospora-associated to na abortion episode over a 1-year period in a dairy herd in a north-east Spain. J Vet Med. 2004; 51(7):348-352.
20. Gondim L, Mineo JR, Schares G. Importance of serological cross-reactivity among Toxoplasma gondii, Hammondia spp., Neospora spp., Sarcocystis spp. and Besnoitia besnoiti. Parasitology. 2017; 144(7):851-868.
21. Raaperi K, Orro T, Viltrop A. Epidemiology and control of bovine herpesvirus 1 infection in Europe. Vet J. 2014; 201(3):249-256.
22. Betancur C, González M, Reza L. Seroepidemiology of infectious bovine rhinotracheitis in the municipality of Montería, Colombia. Rev MVZ Córdoba. 2006; 11(2):830-836.
23. Vargas DS, Bohórquez A, Parra JL, Jaime J, Góngora A. Serological evaluation of bovine herpesvirus 1 and 5 in breeding systems in the Colombian Altillanura. Rev MVZ Córdoba. 2016; 21(2):5381-5389.
24. Camacho R, Carvajal LY, Castellanos-Dominguez YZ, Díaz WF, Vásquez MC. Presence of IgG antibodies against reproductive infections in bulls of the region of Magdalena Medio, Colombia. Rev Colom Cienc Pecua. 2015; 28(4):323-330.
25. Parra J. Influence of bovine viral diarrhea (BVD) virus infection and of bovine leukosis virus, leptospira and infectious bovine rhinotracheitis (IBR) coinfection on milk production. [Master’s Thesis]. National University of Colombia: Colombia; 1994.
26. Peña Cortes LF. Serological study of bovine viral diarrhea in the Cesar Valley microregion. AICA. 2011; 1:309-312.
27. Villamil V. Detection and determination of bovine viral diarrhea virus (BVD) genotypes in four regions of Colombia. [Master’s Thesis]. National University, Faculty of Veterinary Medicine and Animal Science: Colombia; 2015.
28. Schneider MC, Jancloes M, Buss DF, Aldighlieri S, Bertherad E, Najera P et al. Leptospirosis: A silent epidemic disease. Int J Environ Res Public Health. 2013; 10(12):7229-7234.
29. Petrakosvky J, Bianchi A, Fisun H, Nájera-Aguilar P, Pereira MM. Animal leptospirosis in Latin America in the Caribbean countries: Reported outbreaks and literature review (2002-2014). Int J environ Res Public Health. 2014; 11(10):10770-10789.
30. de la Peña-Moctezuma A, Bulach DM, Adler B. Genetic differences among the LPS biosynthetic loci of serovars of Leptospira interrogans and Leptospira borgpetersenii. FEMS Immunol Med Microbiol. 2001; 31(1):73-81.
31. Betancur Hurtado C, Orrego Uribe A, González Tous M. Seroepidemiology of leptospirosis in cattle with reproductive disorders in the municipality of Montería, Colombia. Rev Med Vet. 2013; 26:47-55.
32. Motta JL, Clavijo JA, Waltero I, Abeledo MA. Prevalence of antibodies to Brucella abortus, Leptospira sp. And Neospora caninum in cattle and buffalo herds in the Department of Caquetá, Colombia. Rev Salud Anim. 2014; 36(2):80-89.
33. Zuluaga LAG. Risk factors associated with leptospirosis in bovine herds of Pereira, 2002-2005. Andean Research. 2009; 11(19):120.
34. Polat M, Takeshima SN, Hosomichi K, Kim J, Miyasaka T, Yamada K et al. A new genotype of bovine leukemia virus in South America identified by NGS-based whole genome sequencing and molecular evolutionary genetic analysis. Retrovirology. 2016; 13(4):2-23.
35. Betancur HC, Rods GJ. Seroprevalence of bovine viral leukosis virus in animals with reproductive disorders in Monteria. Rev MVZ Córdoba. 2008; 13(1):1197-1204.
36. Hernández-Herrera DY, Posso-Terranova AM, Benavides JA, Muñoz-Flórez JE, Álvarez-Franco LA. Detection of bovine leukosis virus in Colombian criollo cattle by nested PCR. Agronomic Act. 2011; 60(4):312-318.
37. Carrero Rojas JL. Arévalo Martínez F, Tarazona A, Cepeda BM. Prevalence of seropositivity to bovine leukosis using the indirect ELISA diagnostic technique in dairy herds located in Mesa de los Santos, Santander. Rev Spei Domus. 2009; 5(11):6-11.
38. Úsuga-Monroy C, Echeverri J, López-Herrera H. Molecular diagnosis of bovine leukosis virus in a population of Holstein cows, Colombia. Archives of Animal Science. 2015; 64(248):383-388.
39. Blagitz MG, Souza FN, Batista CF, Azevedo LF, Sanchez EM, Diniz SA. Immunological implications of bovine leukemia virus infection. Res Vet Sci. 2017; 114:109-116.