ORIGINAL
Electrocardiographic parameters in captive, clinically healthy, Amazona ochrocephala
Parámetros electrocardiográficos en Amazona ochrocephala clínicamente sanas en cautiverio
Claudia Guerrero S,1 MV, Janeth Bolivar B,1 MV, Piero Vargas-Pinto,1* MV, Pedro Vargas-Pinto,2 Ph. D, Claudia Brieva-Rico,1 M.Sc.
1Universidad Nacional de Colombia, Facultad de Medicina Veterinaria y Zootecnia, Departamento de Salud Animal, Carrera 30#45. Bogotá, Colombia.
2Universidad de la Salle. Facultad de Ciencias Agropecuarias, Carrera 7° # 172-85. Bogotá, Colombia.
*Correspondence: piavargaspi@unal.edu.co
Received: September 2014; Acepted: February 2015.
ABSTRACT
Objective. To stablish the electrocardiographic parameters of individuals of the species Amazona ochrocephala, from the Unidad de Rescate y Rehabilitacion de Animales Silvestres at the Universidad Nacional de Colombia. Materials and methods. The electrocardiographic examination was performed under inhaled anesthesia with isoflurane. Leads I, II, III, aVL, aVR and aVF were measured. Results. Electrocardiographic parameters obtained in Lead II. P wave Duration: 0.015-0.044 s, P wave amplitude: 0.031 to 0.6 mv, R wave duration: 0.015-0.022 s, amplitude R: 0.034-0.038 mv, S wave Duration: 0.019- 0.042 s, amplitude S: 0.194-0.815 mv, T wave Duration: 0.025-0.064 s, T-wave amplitude: 0.010 to 0.5 mv, PQ Duration: 0.021-0.076 s, QRS Duration: 0.036-0.068 s, QT Duration: 0.070-0.015 s, RR Duration: 0.104-0.324 s, EEM: -111° to -80°, FC: 240-600 ppm. Conclusions. The results showed different values for amplitude and duration of the P, R and T waves in comparison to those obtained in other studies. However, they were similar for heart rate, MEA and duration of the PQ/R, QT and QRS segments.
Key words: Amazona ochrocephala, diagnosis, electrocardiography. (Sources: MeSH).
RESUMEN
Objetivo. Establecer los parámetros electrocardiográficos en individuos de la especie Amazona ochrocephala, de la Unidad de Rescate y Rehabilitación de Animales Silvestres de la Universidad Nacional de Colombia sede Bogotá. Materiales y Métodos. El examen electrocardiográfico se realizó bajo anestesia inhalada con Isoflurano. Se tomaron derivadas I, II, III, aVL, aVR y aVF y posterior medición de ondas e intervalos usando magnificación con lupa. Resultados. Parámetros electrocardiográficos de la segunda derivada. Duración onda P: 0.015-0.044 s, amplitud onda P: 0.031-0.6 mv, duración onda R: 0.015-0.022 s, amplitud R: 0.034-0.038 mv, Duración onda S: 0.019- 0.042 s, Amplitud S: 0.194-0.815 mv, Duración onda T: 0.025-0.064 s, Amplitud onda T: 0.010-0.5 mv, Duración PQ: 0.021-0.076 s, Duración QRS: 0.036-0.068 s, Duración QT: 0.070-0.015 s, Duración R-R: 0.104-0.324 s, EEM: -111° a -80°, FC: 240-600 ppm. Conclusiones. Los resultados mostraron valores diferentes para los rangos de amplitud y duración de las deflexiones P, R y T en comparación a los obtenidos en otros estudios pero similares en cuanto al rango de frecuencia cardiaca, EEM y a la duración de los segmentos PQ/R, QT y QRS.
Palabras clave: Amazona ochrocephala, diagnóstico, electrocardiografía. (Fuentes: MeSH).
INTRODUCTION
The yellow-crowned parrot (Amazona ochrocephala) belongs to the psittacidae family that inhabits forests, mangroves, and tropical forests on the American continent. Although in Colombia this species is in a “least concerned” conservation state, in the country it is one of the wild birds that is most sold due to its demand as a pet, which is an illegal practice (1,2) and they are often caught by the environmental authorities and turned over fauna centers such as the Unidad de Rescate y Rehabilitación de Animales Silvestres (URRAS) of the National University of Colombia.
These birds differ significantly from mammals in their cardiovascular functions (3) and greater research is needed to understand the physiology, pathology and diagnostic methods. Additionally, environmental and nutritional changes of those that are taken to different habitats can alter their normal cardiovascular functioning. One of the most frequently used diagnostic tests in cardiology is electrocardiography (2), and reference values for chickens, turkey, doves and Amazona parrots are available. There is also information (mostly taken from domestic species) on the physiologic and anatomic traits of the cardiovascular system of these birds (3), as well as certain diagnostic procedures on diseases and their treatments. However, there is little information on electrocardiography on Amazona parrots and the authors have not come across studies that report the normal values for electrocardiography parameters for a determined parrot species; a similar study conducted in 1992 took individuals from the Amazona ochrocephala, A. festiva and A. amazonica species to obtain references for ECG parameters (4).
Due to this tendency and the lack of information available concerning normal values for electrocardiographic parameters in this species, the objective of this study was to determine the ECG parameters in Amazona ochrocephala from the Unidad de Rescate y Rehabilitación de Animales Silvestres (URRAS) of the Universidad Nacional de Colombia, Bogotá headquarters.
MATERIALS AND METHODS
Type of study. A non-experimental, transversal type descriptive study was conducted in the period between August and October 2013.
Study site and geoclimate. Unidad de rehabilitación de Animales Silvestres (URRAS), Clínica para Pequeños Animales (CPA) of the Faculty of Veterinary Medicine and Zootechnics, National University of Colombia. Latitude: 4°35’46.3215”N Longitude: 74°4’39.0285”W (5) Altitude: 2560 msnm, average T°: 13.8°C, relative humidity: 73-80% (6); atmospheric pressure 560 mmHg (7).
Study population. Birds from the Amazona ochrocephala species (n=17) kept in individual cages and group enclosures with perches, thermic support and elements to enrich the environment. Fed with a special diet for this species which is composed of fruit, vegetables, seeds, and vitamin and mineral supplements.
Ethic aspects. The study was conducted with the approval of the Bioethics Committee of the Faculty of Veterinary Medicine and Zootechnics, National University of Colombia, in session from August 14, 2013 (Act 06).
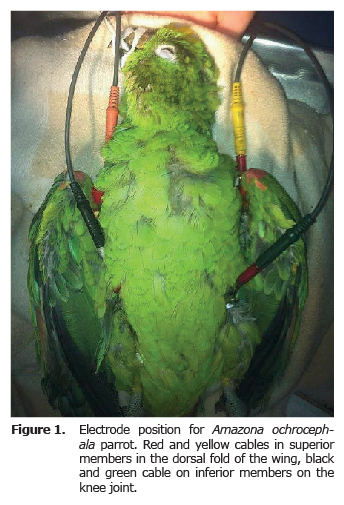
Electrocardiographic record. Before the ECG exam, a basic clinical exam was performed, evaluating the state of hydration, cardiovascular and lung functionality, temperature and weight. For the ECG exam, the animals were put under anesthesia, inhaled with isoflurane between 3-4% for induction (with mask) and 1.5 - 2% for maintenance; tubing was done with endotracheal tube No. 2 or 3 without balloon. With animals in supine position, alligator clips were used for the electrocardiographic procedure, connecting them to the fold of the wings and the thighs (Figure 1). Gel and diluted alcohol was used to improve electric conduction. The ECG record was taken both in bipolar as well as increased unipolar derivatives (ECG Biocare 101G, China) (Figure 2 and 3) with a paper speed of 50 mm/s and an amplitude of 20 mm/mv (8). Anesthesia monitoring was performed by taking heart rate, respiration and temperature. Thermal support was provided during and after the procedure.
Obtaining electrocardiographic parameters. The ECG analysis was performed using five continuous qRs complexes with adequate morphology, measuring each one of the waves and intervals for duration and amplitude in every derivative taken, with the aid of magnifying glass in cases where the deflections were very small (Figure 4). To calculate the mean electric axis, Bailey Axis system was used, taking the sum of the positive and negative deflections (R and S) that represent ventricular depolarization of leads I and III and taking the point where these measurements transpose (9).
Statistical analysis. Data obtained by manual measurements was entered onto a form in Microsoft Excel® version 2010 (Microsoft Corporation®), and a descriptive statistical analysis was compiled. The final results were obtained through averages, standard deviation, minimum and maximum values, which were presented in tables and charts.
RESULTS
The electrocardiograph technique used in this study made it possible to measure the heart rate in all the cases (n= 17), and deflections and intervals in the majority of the parrots. The ECG parameters for lead II are illustrated in tables 1 and 2.
The heart rate was found between 240 y 600 beats/minute with an average of 426 beats/minute (SD 115 beats/minute). (Table 3).
P wave. This wave could only be measured in 76.47% of the parrots (n=13) for lead II, which was the most frequent one. In the majority of cases it was presented as a positive wave, with the exception of the aVL lead in which 33.33% (4 of 12 waves) of the waves were negative.
R wave. This wave was difficult to observe in the ECG tracing, since in the majority of cases it was absent or reading was impossible due to its size. Lead II could only be measured in 17.64% of the cases (n=3 parrots). In aVR and aVL leads it was more difficult to find this wave, in 82.35 and 76.47% of the cases respectively.
S wave. A difficult wave to observe in augmented unipolar leads, in lead II a reliable measurement was taken in 76.47% of the cases (n=13 parrots). There was a negative deflection in all the parrots in all leads.
T wave. For lead II this wave could be measured in 94.11% of the cases (n=16), in the majority as a positive deflection of 93.75% and a negative one of 6.25% in just one parrot. In leads III and aVF, the deflections were completely positive, just the opposite of leads aVR and aVL. For lead I the deflection was mostly positive at 81.25%.
PQ, QT, QRS intervals. These intervals could be measures in 76.47% of the cases (n= 13), for lead II. (Table 2). To discover the QT interval independent of the heart rate, this value was calculated using the Friederichia ![]() value of the corrected QT in Lead II, with an average of 16.365 ms and a standard deviation of 9.893 ms.
value of the corrected QT in Lead II, with an average of 16.365 ms and a standard deviation of 9.893 ms.
R-R intervals. For lead II this interval had an average duration of 168.894 ms with a standard deviation of 56.654 ms, this interval produced similar values in all the leads.
DISCUSSION
Due to being exposed to environmental factors that are different from their habitat, as well as handling in captivity, wild birds are prone to develop cardiovascular abnormalities that can harm their well-being and life (10,11). Changes in temperature, humidity, altitude and pressure, as well as a lack of exercise and inadequate diets, can result in cardiovascular diseases such as arteriosclerosis, pulmonary hypertension and congestive heart failure, among others. Reported heart abnormalities range from 9.7 to 36% (12).
Although ECGs in birds is similar to those used in mammals, there are some differences and chemical restriction or general anesthesia is often required during the procedure, as well as the use of subcutaneous electrodes. The ECG exam under general anesthesia with isoflurane (between 1 and 4%) was safe and a good quality tracing was obtained for the majority of the ECGs recorded, with little interference caused by movements of the animals or electrodes. Also, during the study there were no complications due to anesthesia and postsurgical effects were minimal (emesis and hypothermia).
Regarding the ECG record, the most significant differences have to do with phenomenon such as superimposing T and P waves, the presence of Ta waves, the absence of Q and R waves on some occasions, QRS complexes and short or absent ST segments. Both in presentation such as size and quality in the deflections recorded in the ECG vary in each lead and with each animal. In lead I, the majority of animals presented an isoelectric tracing with very small deflections and complex QRS that were difficult to define.
The aVL lead was more homogenous among all the animals, with regular tracing, good quality and the majority of the deflections were present and easy to measure. As far as the deflections, the P wave in all the leads was too small, making it difficult to get a reliable reading, while the R wave was difficult to obtain in the majority of the bipolar leads, though not so in the augmented unipolar leads where it was frequently recorded. The S wave was absent or difficult to distinguish in the aVR and aVL leads, while in the other leads it was easily identified. Only two birds (lead I and aVL) did not show any deflection that represented ventricular depolarization (R or S wave). On the other hand, the T wave was present in all the leads in almost all the animals examined.
As in other species, infectious and non-infectious diseases have been reported to alter the ECG parameters, causing phenomena such as lengthening waves (P, R, S, T), intervals (PR, TP and QRS), tachycardia and ventricular arrhythmia, premature ventricular contractions, bradycardia and high T waves (13-16). Although the birds used in this study were apparently healthy, it cannot be ruled out that some type of pathology could have affected the ECG results.
In a study done on birds of prey, a wide variability in the morphology of the P wave in lead III was found [positive in 72.5%, negative in 15.7% and biphasic in 11.8% of the animals] (8). In this study, the P wave was always positive in leads I, II, III and aVF, negative in aVR, an positive in 60% of the animals and negative in 40% in aVL. It was also observed that T and P waves were fused or superimposed in 17.6% of the animals (3 of 17 animals) in leads II, III and aVF, and in the majority of recorded ECGs the Q and R waves were absent or too small to be measured.
Finally, it is possible to conclude that a very good quality ECG tracing was obtained when doing the procedure under general anesthesia with Isoflurane (Table 4). Additionally, the results were similar to those reported in the study done by Nap et al (4) with reference to the duration of P, QRS and T waves, heart rate and electric axis, while the amplitude of P, QRS and T waves was lower in our study. However, these differences can be due to the conditions of each study; in the Nap et al (4) study, Amazona ochrocephala, A. amazónica and A. festiva parrots were used, while in this study only Amazona ochrocephala parrots were included, and the conditions of altitude and pressure were not the same in each case, which can affect the parameters.
This study aspires to make more information available about ECGs in Amazona birds in order to facilitate the clinical practice of this important diagnostic tool and thus contribute to the study of physiology and pathologies of the cardiovascular system in these birds.
Acknowledgments
To the Unidad de Rescate y Rehabilitación de Animales Silvestres (URRAS), la Clínica para Pequeños Animales (CPA) of the Universidad Nacional de Colombia-Bogotá, Dr. Milena Lamprea, Dr. Ángela Bermúdez and Dr. José Luis Granados for their participation in this study and for sharing their knowledge with us.
REFERENCES
1. Cruz-Antía D, Gómez JR. Aproximación al uso y tráfico de fauna silvestre en Puerto Carreño, Vichada, Colombia. Ambiente y Desarrollo 2010; 14(26):63-91.
2. Šolc D. The heart and heart conducting system in the kingdom of animals: A comparative approach to its evolution. Exp Clin Cardiol 2007; 12(3):113-118.
3. Richard WN, Couto CG. Pruebas diagnósticas en las enfermedades cardiovasculares. En: Richard WN, Couto CG. Medicina interna de animales pequeños. Cuarta edición. St. Louis, Missouri: ElSevier; 2009.
4. Nap AMP, Lumeji JT, Stokhof AA. Electrocardiogram of the African grey (Psittacus erithacus) and amazon (Amazonas pp.) parrot. Avian Pathol 1992; 21(1):45-53.
5. Instituto Geográfico Agustín Codazzi. Aspectos prácticos de la adopción del marco geocéntrico nacional de referencia MAGNA-SIRGAS como datum oficial de Colombia. Subdirección de Geografía y cartografía del Instituto Geográfico Agustín Codazzi. Bogotá. 2004.
6. López Jiménez VL. Condiciones atmosféricas predominantes en el piedemonte de la cordillera Oriental, determinantes del régimen climático en las sedes de la Universidad de la Salle, Bogotá. Épsilon. 2012; 18:87-104.
7. Hurtado JC, Salazar T, la Peña M de. Valores normales de gases arteriales en Bogotá. Umbral Científico 2007; 10:94-102.
8. Talavera J, Guzmán MJ, del Palacio MJ, Albert AP, Bayón A. The normal electrocardiogram of four species of conscious raptors. Res Vet Sci 2008; 84(1):119-125.
9. Zandvliet MMJM. Electrocardiography in psittacine birds and ferrets. Seminars in Avian and Exotic Pet medicine. 2005; 14(1):34-51.
10. Bavelaar FJ, Beynen AC. Atherosclerosis in parrots. A review. Vet Q 2004; 26(2):50-60.
11. Beaufrère H. Avian atherosclerosis: parrots and beyond. Exotic Pet Medicine 2013; 22(4):336-347.
12. Wit M de, Schoemaker NJ. Clinical approach to avian cardiac disease. Seminars in Avian and Exotic Pet Medicine. 2005; 14(1):6-13.
13. Musulin SE, Adin DB. ECG of the month. Sinus arrhythmia in an african grey parrot. J Am Vet Med Assoc 2006; 229(4):505-507.
14. Nalmas S, Nagarakanti R, Slim J, Abter E, Bishburg E. Electrocardiographic changes in infectious diseases. Hospital Physician. 2007; 43(9):15-27
15. Rembert MS, Smith JA, Strickland KN, Tully TNJr. Intermittent bradyarrhythmia in a hispaniolan amazon parrot (Amazona ventralis). J Avian Med Surg 2008; 22(1):31-40.
16. Knafo SE, Rapoport G, Williams J, Brainard B, Driskell E, Uhl E, et al. Cardiomyopathy and right-sided congestive heart failure in a red-tailed hawk (Buteo jamaicensis). J Avian Med Surg 2011; 25(1):32-39.