LITERATURE REVIEW
Importance of ticks in the transmission of zoonotic agents
Importancia de las garrapatas en la transmisión de agentes causantes de zoonosis
Oscar Betancur H,1* Ph.D, Antonio Betancourt E,2 Ph.D, Cristian Giraldo R,2 MVZ.
1Elanco Animal Health, Transversal 18 No 96-41. Piso 6, Bogotá D.C., Colombia.
2Investigador independiente.
*Correspondence: betancur_oscar_jaime@elanco.com
Received: March 2015; Acepted: July 2015.
ABSTRACT
The pathogens transmitted by ticks to human beings are a motive of public health concern around the world, such is the case of Lyme disease in the northern hemisphere, Encephalitis virus in Europe, the recurrent fevers and the Rocky Mountains spotted fever, better known in Colombia as “Tobia Fever”. People of all economic and social conditions are prone to develop a zoonotic agent transmitted by these vectors, which could be infected by several pathogens through co-infection mechanisms. The epidemiology and prevalence of these diseases are affected by ecological, climatic and anthropogenic factors. All these factors, affect in a different manner the enzootic cycle between pathogens, ticks and wild hosts. Current molecular diagnostic tools have allowed to progress in pathogen identification, previously unknown or undetermined. The government intervention capacity of each country, and the multidisciplinary professional cooperation, especially from physicians and veterinarians, is fundamental in order to strategically implement control and prevention plans that can deal with this problematic. The present article aims to make a thorough review of the factors which are favoring the transmission of zoonotic agents by ticks, contextualizing the most important aspects that determine their prevalence, and the most relevant control and prevention measures.
Key words: Diseases, hosts, pathogens, public health, vectors (Sources: CAB, IEDCYT).
RESUMEN
Los patógenos transmitidos por garrapatas a los seres humanos son motivo de preocupación para la salud pública alrededor del mundo; tal es el caso de la enfermedad de Lyme en el hemisferio norte, el virus de la encefalitis en Europa, las fiebres recurrentes y la fiebre manchada de las montañas rocosas, conocida en Colombia como la “Fiebre de Tobia”. Las personas de todas las condiciones económicas y sociales son susceptibles de contraer un agente zoonótico transmitido por estos vectores, pudiendo ser infectadas incluso por varios patógenos a través de mecanismos de coinfección. La epidemiología y la prevalencia de estas enfermedades se ven afectadas por factores ecológicos, climáticos y antropogénicos. Todos estos factores comprometen de diferente manera el ciclo enzoótico entre patógenos, garrapatas y hospederos silvestres. Las herramientas actuales de diagnóstico molecular han permitido avanzar en la identificación de patógenos anteriormente desconocidos o indeterminados. La capacidad de intervención de los gobiernos de cada país, y la cooperación multidisciplinaria de profesionales, especialmente de médicos y veterinarios es fundamental para implementar planes de control y prevención estratégicos que enfrenten esta problemática. El presente artículo pretende hacer una amplia revisión de los factores que están favoreciendo la transmisión de agentes causantes de zoonosis por las garrapatas, contextualizando los aspectos más importantes que determinan su prevalencia, y las medidas de prevención y control relevantes.
Palabras clave: Enfermedades, hospedadores, patógenos, salud pública, vectores (Fuentes: CAB, IEDCYT).
INTRODUCTION
Zoonoses are diseases and infections naturally transmitted between vertebrate animals and humans. The transmission can be direct, or indirect, by means of vectors (1). Close to 60% of pathogens recognized as causing human disease are zoonotic (2) and the amount of diseases of this type tend to increase (3).
The increase in the appearance of zoonotic pathogens transmitted through ticks is a serious threat to public health worldwide, due to its morbidity and mortality in humans (4). Those of special concern are those that appear for the first time in a population (emergent), or those that have existed and have been labeled under control or extinct, but that quickly increase their prevalence (re-emerging) (2,3). Due to the impact of these diseases on the human population for many decades, the World Health Organization (WHO) and the Pan American Health Organization (PAHO) have formulated guidelines and recommendations in order to advise countries about control of zoonoses (5).
According to Vega (3), it is difficult to know the precise situation of the zoonoses in Colombia because there is no monitoring system designed for such ends; only the casuistry of some zoonoses that are considered priority to the country is gathered; besides, many of the reports of infection are based on the detection of antibodies and, thus, should be considered with caution.
In the present review it is intended to contextualize the factors that facilitate the transmission of tick-borne zoonotic agents, and the key factors that determine its prevalence, as well as the most relevant measures of prevention and control.
Ticks as vectors of zoonotic agents. Only after mosquitos, ticks are considered to be the most important vectors of infectious agents worldwide (6). They are found thoroughly distributed, but their prevalence is greater in countries with warm and humid climates (7). The majority of species of ticks occupy habitats such as forests, savannahs, grasslands and scrublands, in which they can survive long periods of time until they find a host on which to feed (8).
Ticks are arthropods belonging to the Arachnid class (9). They are a diverse group, with at least 896 recognized species, distributed into three families: Argasidae or “soft ticks” (193 spp), Ixodidae or “hard ticks” (702 spp), and only one species belonging to the Nuttalliellidae family (10).
Ticks are important vectors of various pathogenic bacteria, such as protozoans and viruses that cause diseases in animals and humans (11). These microorganisms have evolved to take advantage of the life cycle of ticks, using them as a means to disperse from one vertebrate host to another (8). The life cycle of ticks, which include consumption of host blood, the secretion of saliva into the tissue of the host, the movement between different hosts, and the production of eggs from which a new generation of ticks develops that inevitably makes them an adequate home for other organisms (12).
Transmission of pathogens. Some species of ticks can transmit pathogens from wild animals (for example, rodents) to humans (9); the transmission can also be made through domestic animals (7). The risk of transmission is determined by the prevalence of ticks in the environment and by the probability of an encounter between an infected tick and a susceptible host (9).
The transmission of pathogens requires, in the first place, that during feed-uptake, the tick acquires the pathogens of an infected vertebrate host (13). Ticks also can transmit infectious agents from an infected tick to another by co-feeding at the same site in the host, even when the latter is not infected (14); transovarial and/or trans-stadial transmission is an important trait (15). Evolutionally, ticks have developed a protective response to limit levels of pathogen infection, which contribute to their survival (16).
Secondly, pathogenic agents must survive to the development and change between stages of the tick, and become established in the salivary glands of the tick for transmission during the next feeding (13). In this way, some studies indicate that the prevalence of infection in ticks by pathogenic agents is reduced dramatically when there is a change of stage in the tick (15).
Finally, hosts should be competent enough to allow the establishment, the replication and the survival of the infected agents (13). Hosts normally acquire the infection through infected saliva when the ticks are feeding, but in addition, other routes of transmission include infection through the consumption of infected ticks, the manipulation of the tissue of dead infected animals, inhalation, ingestion of non-pasteurized milk and artificial infection through blood transfusion, this latter transmission being of special attention in human and veterinary medicine (12).
Many ticks can house two or more infected agents and transmit them simultaneously (14, 17). This phenomenon is especially frequent in the species of tick known as Ixodes ricinus, which has non-specific feeding habits and feeds on a great variety of vertebrate species that are reservoirs for multiple pathogens (18, 19). Alternatively, hosts can be co-infected when bitten by different ticks and at different times (12). Co-infection with multiple pathogens occurs frequently, which causes a serious challenge for adequate diagnosis and treatment (18). On the other hand, even though some pathogens can be transmitted by the same tick (example, Borrelia burgdorferi sensu lato, Anaplasma phagocytophilum and Rickettsia helvetica), they have different ecological cycles and transmission patterns that influence the prevalence of the infection in the different life stages of the tick (20).
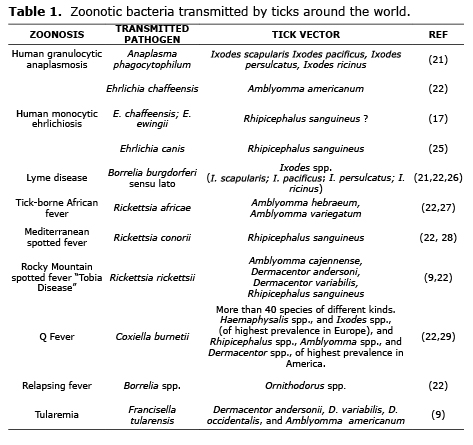
Most important pathogens causing tick-borne zoonoses. Worldwide, the number of different and epidemiologically important diseases recognized as caused by tick-borne pathogens has considerably increased during the las 30 years (16). For example, Lyme disease in North America and Europe (21); the encephalitis virus transmitted by ticks in Europe and Asia; relapsing fevers in Africa, North America, Europe and Asia (22); and the Rocky Mountain spotted fever in all of the America, known in Colombia as the “Tobia Fever”, all have recorded instances of fatality (23).
In Europe, ticks are the principle vectors of zoonotic agents (24), which present a challenge for public health because of its great impact on human health and world economy (8). Some important zoonotic agents transmitted by ticks can be found in tables 1, 2 and 3.
Ecological factors associated with tick-borne zoonotic agents (TBZA). TBZAs typically appear because of interference in circulation between animals, wild reservoirs and ticks (12). Even though it is considered that wild animals are the principle reservoirs of various pathogens that cause disease in humans, for some TBZAs, domestic animals can eventually become important reservoirs for human pathogens (34).
The displacement of ticks is limited, and they cannot be displaced over great distances without the help of a vertebrate that acts as a mechanical vector with which ticks can cross natural boundaries and be dispersed over mountain chains, islands and bodies of water (35); ticks also colonize new territories when mechanical vectors are introduced by humans in new areas (12). After arriving to a new territory, the survival of the ticks is limited by the presence of other competitors, predators and the availability of hosts (35).
The most competent natural reservoirs for some tick-borne pathogens are hosts that tend to have high population densities and rapid life cycles (36); such as rodent and birds (20). In the same way, rodents constitute one of the most important hosts for ticks, especially for larvae and nymphs. Nonetheless, the microclimatic conditions of a particular zone can affect contact between ticks and rodents, which can influence the selection of new hosts (37).
For their part, birds have be found to be carriers of some species of ticks (16), in which case migratory birds are particularly important, since the presence of Bartonella spp. (38), Anaplasma sp., Borrelia sp., and Rickettsia sp., have already been proven in ticks obtained from birds (39). Birds are not simple mechanical vectors; they can develop an infection, which converts them into active pathogen hosts, for example, the developing of bacteremia such as Rickettsia helvetica and Anaplasma phagocytophilum. The prevalence of tick-borne infestations in birds is associated to a large degree with the habit of ground feeding (40).
Birds have great epidemiological importance due to the fact that they can fly great distances in the course of only a few days (40). Epidemiologic potential is greater because of horizontal transmission of TBZAs between birds that share ecological niches (39).
The increase in interaction between wild and domestic animals has increased the transmission of ticks to humans (7). In regards to canines, they can serve as reservoirs for human pathogens, as definitive hosts or mechanical transporters of ticks, and as sentinel indicators of regional disease risk (9). Although the majority of species of ticks are of a singular host, the introduction of infected larvae and nymphs to a home can cause the transmission of pathogens to humans after the tick’s change of stage inside the house. On the other hand, the introduction of an engorged female can facilitate the establishment of a population of ticks inside the home, some of which are able to successfully adapt to the conditions of human residences (as R. sanguineus) (34).
Anthropogenic factors associated with TBZAs. One would think that humans were one of the most common food sources for ticks having in mind the huge size of human population in the world, its great density in some areas, and the size of a human adult’s body; however, they are not important reservoirs for TBZAs, although ticks can potentially cause sickness in a great number of individuals, such episodes typically are an accident (12).
Over a long period of time, all countries have experienced important changes in their epidemiological profile as a result of social, economic and cultural transformations (5). In recent years, many factors have changed the interactions between humans, animals and the environment, which has caused the emergence and re-emergence of many diseases (7). The appearance of AZTGs is becoming an increasing problem due to the intensification of migrations of humans and animals, and environmental changes (18). In effect, human populations are growing and expanding in new geographic areas, invading wild ecological niches and facilitating the transmission of pathogens to humans (41).
The emergence of tick-borne zoonotic diseases is associated with changes in the use of the soil, which obligate pathogens and ticks to find new transmission mechanisms associated with humans; on the other hand, the population’s social economic conditions also facilitate the emergence of these diseases; the risk is greater for people with high and low incomes than those of the middle class. For example, people of higher income look for country homes near forests (42), or they have the possibility of travelling to previously inaccessible places; in addition, the popularity of out-door activities such as hiking and fishing has increased (14). On the other hand, economically disadvantaged people can be less protected against infection (because of malnutrition or immune suppression). In addition, access to available vaccines for some AZTGs, which are usually expensive, is limited (43).
Climatic factors associated with TBZAs. Without a doubt, climate change has a strong impact on the dynamics of infectious diseases (5). Climate directly affects the livelihood of ticks (16) and can also indirectly affect them through the effect it has on vegetation and hosts, as much in terms of abundance as survival (26).
Microclimatic variables such as the temperature of ground surface and relative humidity can be crucial in determining the distribution pattern of specific niches and the survival of ticks in a given area (26). The abundance of ticks is greater in lower altitudes, where the climate is warmer. At high altitudes where the temperatures are lower, ticks are less active, which reduces their access to hosts, because of which they can have a lesser probability of survival than ticks in low altitudes (24). The decreased activity of ticks in cold environments lowers pathogen transmission (16).
Climate change can facilitate the migration of vertebrate hosts, which permits the spread of ticks and pathogens to new territories (12); because of weather changes, these new areas can be transformed into favorable habitats for ticks (for example, the warmest temperatures in the highest elevations or latitudes) (9). Another point-of-view indicates that, although climate change can result in adequate climate conditions in some places, these zones will remain free of the disease unless all components of the system (host, vector and pathogen) arrive and are simultaneously sustained in these locations during a period of time sufficient for establishing transmission cycles (35).
In common with almost all arthropods, development rates of ticks increases with the rise of temperature (13). Specifically, the highest temperatures raise three fundamental aspects: the vector’s bite rate, development rate and the rate of pathogen replication; none the less, frequently they diminish the survival of the vector, especially when it is associated with a lack of humidity (42). The combined effects of temperature and humidity influence the behavior, survival and reproduction of many species of vectors. The same conditions limit the ability of ticks to find hosts in exposed environments (35). In addition, relatively prolonged periods of high temperatures and low humidity can interrupt the enzootic cycle of some tick-borne pathogens, for example that of tick-borne encephalitis (37).
Although the impact of climate change has been supported on the basis of the rise in temperature, the potential influence of changes in rainfall patterns has frequently been ignored, even when this could have a greater effect than temperature on the ability of tick populations to establish themselves in new zones (16)
Diagnosis. The prevalence or the report of zoonotic diseases from tick-borne pathogens has increased in the past years, in great measure because of advancements made in diagnostic tools, especially in molecular techniques which have permitted laboratories to increase the ability of detecting new pathogens and distinguish between microorganism species and strains (12). Techniques such as the polymerase chain reaction (PCR) have allowed simultaneous identification of different types of viruses, in addition to bacteria that are difficult to grow in traditional culture mediums, or that have been found in samples that have been treated with antibiotics. Nonetheless, the use of serology, cultures and histopathology among others, continue to be tools of vital importance in identifying zoonotic agents (44).
However, the diagnostic ability of some countries like Colombia can be limited, where an adequate network of zoonosis detection laboratories that facilitate the adequate process and the confirmation of these diseases is lacking; also, diagnostic and epidemiological methods are unknown (3). In the same way, it is presumed that some cases of zoonosis are processed sub-clinically and thus never diagnosed (45).
On the other hand, important advances in epidemiology studies have been made. The use of multivariate analysis has facilitated a better understanding of all the components that are part of the complex cycles involving ticks, reservoirs, and pathogens (35).
Multi-parametric models have made it possible to predict and estimate changes in geographic distribution areas of ticks (12). The most commonly used strategy for estimating the potential geographic distribution area of a species is the characterization of environmental conditions that are adequate for the species, and later identifying where the right environment exists (16). Although simple predictions about changes in tick abundance can be made, it is necessary to make similar predictions about the availability of appropriate hosts coinciding with the population increase of ticks over space and time (24).
Control and prevention. Zoonotic diseases caused by tick-borne pathogens can be difficult to control due to the complex epidemiology that involves different ticks and hosts (34). For this reason, the fundamental tool for control is permanent epidemiological monitoring. Programs for control of these diseases requires the integration of data from veterinary and human information systems, the monitoring of wild animals and tick populations, and teams of experts from various scientific disciplines such as entomology, epidemiology, medicine, public health and veterinary medicine (12). Government agencies in charges of public health are fundamental as a base for information for all epidemiological studies (46). A database has been developed that involves ticks as vectors for zoonotic pathogens within a project financed by the European Union, with the collaboration of SAPUVETNET III, in which not only information about this subject is offered, but predictive maps of geographic distribution of ticks and zoonotic diseases caused by tick-borne pathogens (4).
Principles, strategies and control and prevention tactics for TBZAs in humans should be adapted to the conditions of each country (3). It is imperative to develop measures of integrated control, which include: vectors control, vaccination programs against tick-borne zoonotic agents, betterment of treatment strategies, improvement of diagnostic and monitoring equipment, and public awareness campaigns (47).
The principal method for controlling ticks is the application of chemical compounds. The problem with this is that it requires frequent reapplication, which is a risk to the health of non-objective, incidentally exposed animals (9). On the other hand, the constant reapplication can cause resistance to the compound; in addition, very little is known about the degree to which biocides and their toxic degradation products are accumulated in predators, scavenger birds, and mammals (8); in the same way, a control strategy based exclusively on the use of acaricides, implies risks to humans because of the danger of deposition of pesticide residues in products and sub-products of animal origin (such as meat, milk and eggs), in addition to being a contaminate to the environment. The direct application of acaricides to vertebrate hosts of ticks reduces the effects on non-objective species, but should be included in a strategic control plan (48). This plan should include the rotation of products by action mechanisms of their active ingredients and not by their commercial names, for example organophosphate and pyrethroid insecticides alternated with fluazuron as a quitine inhibitor.
When also turning to non-chemical control as part of an integrated plan, it becomes and important complementary alternative to chemical compounds; normally they are not toxic to non-objective humans or animals, and reduce the risk of forming resistance. These include the use of natural predators of ticks such as insectivorous birds, unfavorable hosts, parasitoid wasps, nematodes, bacteria and entomopathogenic fungi. The latter in particular have proven to be very effective in controlling all types of ticks: Rhipicephalus (Boophilus spp.), and Amblyomma spp. associated with livestock (8).
Human vaccination programs against tick-borne zoonotic agents are very effective but have some limitations. On one hand, the cost makes them unavailable to the entire population, making governmental assistance through public health authorities necessary (43); on the other hand, vaccines for the majority of tick-borne pathogens do not exist (32); only some such as tick-borne encephalitis (1) or that of Lyme disease. It is important to understand that vaccination against specific pathogens does not omit the need for other measures to prevent tick bites, being that the vaccine does not give cross protection against other TBZAs (9).
Educating pet owners on the implementation of integrated tick control programs could be crucial in reducing the risk of infestation and can decrease the probability of bringing infected ticks into homes. For people who visit or work in environments with tick infestations, the use of tick repellent and acaricide is advised (34).
In summary, preventative measures should be taken and strategic short, medium and long-term control plans should be adopted. The most effective preventative measures are: immunoprophylaxis, if a vaccine exists, and use of protective clothing and repellent. In areas that are natural focal points for ticks (such as natural parks, reserves and city suburbs), preventative measures are centered on: establishing barriers for the vector, eliminating feeding sites close to human settlements and applying insecticides to surrounding areas (3). It should be understood that no technique alone is 100% effective; nonetheless, a program should be chosen that integrates various preventative components to maximize protection against TBZAs (9).
Research perspectives. The Colombian panorama in terms of epidemiological studies regarding TBZAs is large. Demographic expansion in many cases forces invasion into diverse ecological niches; for this reason, organizations that manage land development plans should consider epidemiological studies of all zones that can be focal points of infection; requiring that investigations are made to facilitate the identification of possible TBZAs and risk factors associated with a particular zone.
Activities such as ecotourism, hiking and fishing, among others, can be a risk factor for contracting TBZAs; it is necessary to carry out studies which evaluate risk factors and can implement prevention and control measures in places where these activities are promoted, especially, when the end of the Colombian armed conflict ends in the near future, which will promote in great measure the increase of these forms of entertainment.
Multifactorial studies can be carried out in which the effects on TBZAs on climate change in Colombia are considered, having in mind variables such as temperature, relative humidity and rainfall, in addition to current tick species and the availability of native hosts as well as potential said arthropods. Another possible investigation can be made to assess migratory birds that arrive to Colombia and ticks that they carry, where the role that these vertebrates play in the spread of zoonotic diseases caused by tick-borne pathogens in this nation can be established.
REFERENCES
1. Dobler G. Zoonotic tick-borne flaviviruses. Vet microbiol 2010; 140(3-4):221-8.
2. Monsalve S, Mattar S, Gonzalez M. Zoonosis transmitidas por animales silvestres y su impacto en las enfermedades emergentes y reemergentes. Rev MVZ Córdoba 2009; 14(2):1762-73.
3. Vega RL. Zoonosis emergentes y reemergentes y principios básicos de control de zoonosis. Rev Med Vet Zoot 2009; 17(enero-junio):85-97.
4. Vesco U, Knap N, Grindatto A, Avsic-Zupanc T, Labruna M, Estrada-Peña A, et al. A database on ticks and tick-borne zoonoses in the (sub-)tropics: an example of intersectoral and institutional collaboration at international level. Una Salud Rev Sapuvet Sal Púb 2010; I(enero-junio):41-8.
5. Agudelo-Suárez AN. Aproximación a la complejidad de las zoonosis en Colombia. Rev Salud Pública 2012; 14(2):325-39.
6. Toledo A, Olmeda AS, Escudero R, Jado I, Valcarcel F, Casado-Nistal MA, et al. Tick-borne zoonotic bacteria in ticks collected from central Spain. Am J Trop Med Hyg 2009; 81(1):67-74.
7. Liyanaarachchi DR, Rajakaruna RS, Dikkumbura AW, Rajapakse RP. Ticks infesting wild and domestic animals and humans of Sri Lanka with new host records. Acta Trop 2015; 142(Feb):64-70.
8. Ostfeld RS, Price A, Hornbostel VL, Benjamin MA, Keesing M. Controlling Ticks and Tick-borne Zoonoses with Biological and Chemical Agents. BioScience 2006; 56(5):383-94.
9. Fritz CL. Emerging tick-borne diseases. Vet Clin North Am Small Anim Pract 2009; 39(2):265-78.
10. Guglielmone AA, Robbins RG, Apanaskevich DA, Petney TN, Estrada-Peña A, Horak I, et al. The Argasidae, Ixodidae and Nuttalliellidae (Acari: Ixodida) of the world: a list of valid species names. Zootaxa 2010; 2528(06):1-28.
11. Barandika JF, Hurtado A, Garcia-Sanmartin J, Juste RA, Anda P, Garcia-Perez AL. Prevalence of tick-borne zoonotic bacteria in questing adult ticks from northern Spain. Vector Borne Zoonotic Dis 2008; 8(6):829-35.
12. Baneth G. Tick-borne infections of animals and humans: a common ground. Int J Parasitol 2014; 44(9):591-6.
13. Randolph SE. Tick-borne disease systems. Rev Sci Tech Off Int Epiz 2008; 27(2):1-15.
14. Mansfield KL, Johnson N, Phipps LP, Stephenson JR, Fooks AR, Solomon T. Tick-borne encephalitis virus - a review of an emerging zoonosis. J Gen Virol 2009; 90(Pt 8):1781-94.
15. Slovak M, Kazimirova M, Siebenstichova M, Ustanikova K, Klempa B, Gritsun T, et al. Survival dynamics of tick-borne encephalitis virus in Ixodes ricinus ticks. Ticks Tick Borne Dis 2014; 5(6):962-9.
16. Estrada-Peña A, Ayllon N, de la Fuente J. Impact of climate trends on tick-borne pathogen transmission. Front Physiol 2012; 3(Mar):64.
17. Ndip RN, Ndive VE, Awuh JA, Walker DH, McBride JW. Ehrlichia species in Rhipicephalus sanguineus ticks in Cameroon. Vector Borne Zoonotic Dis 2007; 7(2):221-7.
18. Reis C, Cote M, Paul RE, Bonnet S. Questing ticks in suburban forest are infected by at least six tick-borne pathogens. Vector Borne Zoonotic Dis 2011; 11(7):907-16.
19. Lommano E, Bertaiola L, Dupasquier C, Gern L. Infections and coinfections of questing Ixodes ricinus ticks by emerging zoonotic pathogens in Western Switzerland. Appl Environ Microbiol 2012; 78(13):4606-12.
20. Halos L, Bord S, Cotte V, Gasqui P, Abrial D, Barnouin J, et al. Ecological factors characterizing the prevalence of bacterial tick-borne pathogens in Ixodes ricinus ticks in pastures and woodlands. Appl Environ Microbiol 2010; 76(13):4413-20.
21. Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2006; 43(9):1089-134.
22. Piesman J, Eisen L. Prevention of tick-borne diseases. Annu Rev Entomol 2008; 53:323-43.
23. Quintero JC, Hidalgo M, Rodas JD. Rickettsiosis, una enfermedad letal emergente y re-emergente en Colombia. Univ Sci 2012; 17(1):82-99.
24. Gilbert L. Altitudinal patterns of tick and host abundance: a potential role for climate change in regulating tick-borne diseases? Oecologia 2010; 162(1):217-25.
25. Perez M, Bodor M, Zhang C, Xiong Q, Rikihisa Y. Human infection with Ehrlichia canis accompanied by clinical signs in Venezuela. Ann N Y Acad Sci 2006; 1078:110-7.
26. Gray JS, Dautel H, Estrada-Pena A, Kahl O, Lindgren E. Effects of climate change on ticks and tick-borne diseases in europe. Interdiscip Perspect Infect Dis 2009; 2009:593232.
27. Althaus F, Greub G, Raoult D, Genton B. African tick-bite fever: a new entity in the differential diagnosis of multiple eschars in travelers. Description of five cases imported from South Africa to Switzerland. Int J Infect Dis 2010; 14 Suppl 3:e274-6.
28. Rovery C, Raoult D. Mediterranean spotted fever. Infect Dis Clin North Am 2008; 22(3):515-30, ix.
29. Contreras V, González M, Guzmán C, Máttar S. Fiebre Q: una zoonosis olvidada en Colombia. Rev Méd Risaralda. 2013; 19(2):137-46.
30. Brackney MM, Marfin AA, Staples JE, Stallones L, Keefe T, Black WC, et al. Epidemiology of Colorado tick fever in Montana, Utah, and Wyoming, 1995-2003. Vector Borne Zoonotic Dis 2010; 10(4):381-5.
31. Ergonul O. Crimean-Congo haemorrhagic fever. Lancet Infect Dis 2006; 6(4):203-14.
32. Liu Q, He B, Huang SY, Wei F, Zhu XQ. Severe fever with thrombocytopenia syndrome, an emerging tick-borne zoonosis. Lancet Infect Dis 2014; 14(8):763-72.
33. Gray J, Zintl A, Hildebrandt A, Hunfeld KP, Weiss L. Zoonotic babesiosis: overview of the disease and novel aspects of pathogen identity. Ticks Tick Borne Dis 2010; 1(1):3-10.
34. Dantas-Torres F, Chomel BB, Otranto D. Ticks and tick-borne diseases: a One Health perspective. Trends Parasitol 2012; 28(10):437-46.
35. Mills JN, Gage KL, Khan AS. Potential influence of climate change on vector-borne and zoonotic diseases: a review and proposed research plan. Environ Health Perspect 2010; 118(11):1507-14.
36. Ostfeld RS, Levi T, Jolles AE, Martin LB, Hosseini PR, Keesing F. Life history and demographic drivers of reservoir competence for three tick-borne zoonotic pathogens. PLoS One 2014; 9(9):e107387.
37. Burri C, Bastic V, Maeder G, Patalas E, Gern L. Microclimate and the zoonotic cycle of tick-borne encephalitis virus in Switzerland. J Med Entomol 2011; 48(3):615-27.
38. Molin Y, Lindeborg M, Nystrom F, Madder M, Hjelm E, Olsen B, et al. Migratory birds, ticks, and Bartonella. Infect Ecol Epidemiol 2011; 1:5997.
39. Palomar AM, Santibanez P, Mazuelas D, Roncero L, Santibanez S, Portillo A, et al. Role of birds in dispersal of etiologic agents of tick-borne zoonoses, Spain, 2009. Emerg Infect Dis 2012; 18(7):1188-91.
40. Hornok S, Kováts D, Csörgõ T, Meli ML, Gönczi E, Hadnagy Z, et al. Birds as potential reservoirs of tick-borne pathogens: first evidence of bacteraemia with Rickettsia helvetica. Parasit Vectors 2014; 7:128.
41. Cabello C, Cabello F. Zoonosis con reservorios silvestres: Amenazas a la salud pública y a la economía. Rev Méd Chile 2008; 136(3):385-93.
42. Kilpatrick AM, Randolph SE. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012; 380(9857):1946-55.
43. Godfrey ER, Randolph SE. Economic downturn results in tick-borne disease upsurge. Parasit Vectors 2011; 4:35.
44. Fenollar F, Raoult D. Molecular diagnosis of bloodstream infections caused by non-cultivable bacteria. Int J Antimicrob Agents 2007; 30(Suppl. 1): 7-15.
45. Fuentes M, Pérez L, Suárez Y, Soca M, Martínez A. La zoonosis como ciencia y su impacto social. Rev Electr Vet 2006; 7(9): 1-19.
46. De Meneghi D. Wildlife, environment and (re)-emerging zoonoses, with special reference to sylvatic tick-borne zoonoses in North-Western Italy. Ann Ist Super Sanita 2006; 42(4):405-9.
47. Ahmed J, Bouloy M, Ergonul O, Fooks A, Paweska J, Chevalier V, et al. International network for capacity building for the control of emerging viral vector-borne zoonotic diseases: ARBO-ZOONET. Euro Surveill 2009; 14(12. 1-4.).
48. Piesman J, Beard CB. Prevention of tick-borne diseases. J Environ Health 2012; 74(10):30-2.