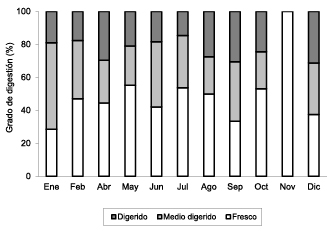
Figure 1. Degree of monthly digestion.
ORIGINAL
Feeding habits of Cocobolo Andinoacara pulcher in the cienaga Grande de Lorica, Colombia
Hábitos alimentarios de Cocobolo (Andinoacara pulcher) en la ciénaga Grande de Lorica, Colombia
Charles W. Olaya-Nieto,1 M.Sc, Liquey Camargo-Herrera,2 Prof. Acuicul, Vanessa Díaz-Sajonero,2 Prof. Acuicul, Fredys F. Segura-Guevara,1* M.Sc.
1Universidad de Córdoba, Faculty of Veterinary Medicine and Zootechnical Science, Department of Aquatic Sciences, Laboratory of Biological Research LIBP. Cra 23 No. 2A-20, Piso 2. Lorica, Colombia.
2Universidad de Córdoba, Faculty of Veterinary Medicine and Zootechnical Science, Department of Aquatic Sciences, Aquaculture Program. Montería, Colombia.
*Correspondence: ffsegura@correo.unicordoba.edu.co
Received: June 2014; Accepted: April 2015.
ABSTRACT
Objective. he feeding habits of Cocobolo (Andinoacara pulcher) in the cienaga Grande de Lorica, Sinu river basin, were studied. Materials and methods. The stomach content was analyzed using the Proportion of empty stomachs, Grade of digestion, Frequency of occurrence, numerical Frequency, Gravimetry, relative importance Index (RII) and the gut length-total length relationship. Results. 39.8% of stomachs were empty, 47.1% of preys were fresh and five food groups were identified. Vegetable remains was the most frequent group (63.8%) and the prey with greatest composition in weight (33.5%), while Rest of fishes was the most abundant group (34.7%). It was observed that in low and rising waters, fishes was the most consumed prey, while that in high and falling waters the most consumed prey was vegetable remains. Vegetable remains, detritus and fishes were food groups of secondary relative importance, while Insects and Others were circumstantial or incidental groups. Conclusions. The results achieved indicate that Cocobolo is a fish with omnivores feeding habits with a preference for vegetable remains.
Key words: Diet, feeding, food preferences, trophic levels (Source: CAB).
RESUMEN
Objetivo. e estudiaron los hábitos alimentarios de Cocobolo (Andinoacara pulcher) en la ciénaga Grande de Lorica, cuenca del río Sinú, Colombia. Materiales y Métodos. El contenido estomacal se evaluó con el Coeficiente de vacuidad, Grado de llenado, Grado de digestión, Frecuencia de ocurrencia, Frecuencia numérica, Gravimetría, Índice de importancia relativa y la relación longitud intestinal-longitud total. Resultados. El 39.8% de los estómagos se encontró vacío, el 47.1% de las presas en estado fresco y se identificaron cinco grupos alimentarios. Material vegetal fue el grupo más frecuente (63.8%) y con mayor composición por peso (33.5%), mientras que Restos de peces fue el más abundante (34.7%). Se observó que en aguas bajas y en aguas ascendentes, peces fue la presa más consumida, mientras que en aguas altas y aguas descendentes, fue material vegetal. Material vegetal, detritos y restos de peces fueron grupos alimentarios de importancia relativa secundaria, mientras que insectos y otros fueron circunstanciales o incidentales. Conclusiones. Los resultados alcanzados indican que Cocobolo es un pez de hábitos alimentarios omnívoros con preferencia por material vegetal.
Palabras clave: Alimentación, dieta, niveles tróficos, preferencias alimentarias (Fuente: CAB).
INTRODUCTION
Cocobolo (Andinoacara pulcher) (1) is a small fish that has sedentary habits. The males are more colorful and larger than the females, and turn a more vibrant blue in the reproductive season.
In Colombia, they are found in the rivers of the Caribbean versant, including the Sinú river basin, as in the case of the Ciénaga Grande de Lorica, where it generally does not form part of the commercial fishery. However, both in the swamp as well as in the basin, it is an important component in the food chain, because it is one of the most consumed fish by large and small predators, such as Ariopsis sp. (2) and Rhamdia quelen (3), respectively.
Studies in other geographical areas of the country report that the species consumes worms, crustaceans, insects and live preys (4). On the Ciénaga Grande de Lorica, The length at first maturity is 9.5 cm LT, with prolonged spawning season that extends throughout the year with partial spawning of 954 oocytes (5).
The objective of this study was to determine the feeding habits of Cocobolo on the Ciénaga Grande de Lorica as a contribution to knowledge of its biology and ecology and its preservation in the environment and the management of their fishery in the floodplain and in the Sinú basin.
MATERIALS AND METHODS
Study area. This study took place in Ciénaga Grande de Lorica, a body of water located downstream from the Urrá dam, which has a maximum depth of 5 meters in the rainy season. Multi-year average rainfall reaches 1200 mm with a bimodal rainfall and an average temperature of 27 °C from the study area to the coastal area of the Caribbean sea (6).
Obtaining samples. Musilová et al (1) was followed for species identification. 294 individuals were captured between January and December 2005, except in March, and total length (TL) and standard length (SL) was measured to the nearest cm with a graduated measuring ictiometer (IK2, Aquatic Biotechnology, Spain) and total weight was measured (TW) with an electrical scale 5000 ± 1 g (CS 5000, Ohaus Corporation, USA). The fish were caught with hook and line and castnet, kept cool in 51 liter polyurethane coolers (Cooler Camping 2A16, Rubbermaid, USA) and were transported to the Fisheries Biology Research Laboratory-FBRL of the Universidad de Córdoba in Lorica.
Stomach extraction. Following techniques used by Laevastu (7), the fish were dissected and different parts of the digestive tract were located. The stomach and intestines were then removed and stored in plastic bottles containing 250 ml of buffered formalin at 10%. All bottles were labelled to indicate species, sample number and date. The above mentioned information was recorded as well as the site of capture, fishing gear used, size, total weight and gutted weight.
Analysis of stomach content. Stomach content was placed in a Petri dish and was examined with a stereoscope (Leica Zoom 2000, Leica Microsystems GmbH, Germany) and microscope (Leica CME, Leica Microsystems GmbH, Germany), separating, identifying and numbering the food or preys present. Identification was performed until the taxonomic level allow according to the degree of digestion of the food, organizing the information by items, preys or groups, and weighing them on an 1500 ± 0.01 g electric scale (Adventurer, Ohaus Corporation, USA).
The emptiness coefficient (CV) was estimated as CV = 100* Number of empty stomachs / Total number de analyzed stomachs (8).
The degree of digestion (GD) was evaluated according to the state of fresh, partially digested and digested preys (7).
Three methods were used to quantify stomach contents, expressed in average monthly and annual values: frequency of occurrence (FO), numeric frequency (FN) and gravimetrics (G) (9).
FO = 100* Occurrence of preys from item A/Total number of stomachs with food.
FN= 100* Number of preys from item A/Total number of prey.
G = 100* Weight of preys from item A/Weight of all prey.
To establish the importance of each prey in the diet, the modified relative importance index (RII) was estimated (10): RII = F * G / 1OO where RII represents the relative importance index, F is the frequency of occurrence percentage and G is the weight percentage. This is expressed in a range from 0 to 100, where 0 to 10% represents trophic groups with relatively low importance, 10 to 40% as relatively important and 40 to 100% as groups of relatively high importance.
The total length gut-length relation was established according to the Brusle scale (11) and food preferences were analyzed according to the sizes of the species under study and the water cycle of Ciénaga Grande de Lorica in seven class intervals (7.5-8.5 to 13.5-14.5 cm LT) and taking into account the classification used to define the four levels of Cienaga Grande de Lorica during its annual water cycle: low waters (December, January, February), rising waters (March, April, May), high waters (June, July, August) and falling waters (September, October, November).
Descriptive statistics were used, expressing variables as mean ± standard deviation, and a correlation coefficient for the intestinal length- total length relationship was estimated.
RESULTS
The minimum recorded size was 7.2 cm TL (March), the maximum was 14.5 cm LT (October and December) and the lowest weight was 9.1 g (March) and the highest 89.1 g (December). The frequency distribution for sizes showed a curve with ranges between 7.0 and 15.0 cm LT, and an average catch size of 10.7 cm LT (n=294) that indicates that 17.3% of individuals were caught below the length at first maturity for both sexes as estimated by Olaya-Nieto et al (5), presenting sexual dimorphism in size given that males are larger than females. The frequency distribution for weight showed ranges between 10.0 and 90.0 g, an average weight of 30.0 g and modal frequency at 20.0 g.
39.8% of stomachs studied were empty, especially in the months of March (100.0%), November (70.0%), December (64.3%), February (56.5%) and April (50.0%), where the maximum values were reached. 47.1% of food consumed was fresh, 29.8% half-digested and 23.1% digested. As shown in figure 1, fresh prey was found in almost every month except March, when all stomachs were empty (CV=100%), reaching highest monthly values except in January (52.4%) and September (36.1%), where the predominant state was half-digested prey. It is noteworthy that in November there was an absence of half-digested (0.0%) and digested states (0.0%).

Figure 1. Degree of monthly digestion.
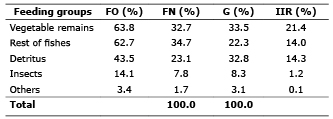
Five prey or food groups were identified: Vegetable remains, fishes, detritus, insects and other types, which showed the following annual frequency occurrence: Vegetable remains (63.8%) was the most common; fishes (62.7%) including scales, spines and fins; detritus (43.5%); insects (14.1%) including Hymenoptera, insect rest and Hymenoptera eggs and other types (3.4%), including snails, worms and nylon. Vegetable remains and fishes were present in every month of the year except March, while detritus was found in every month except March and November, insects were absent in July and November and other types were present in January, May, August and October.
The most abundant item or group was fishes (34.7%), which was present every month, followed by Vegetable remains (32.7%), detritus (23.1%), insects (7.8%) and other types (1.7%). As in the frequency of occurrence, almost all these items were found throughout the year except in November, when no detritus, insects or other types were found. In greater detail, Vegetable remains was more abundant in five months of the study, especially in July (48.8%) and November (60.0%); fishes were more abundant in six months of the year, mainly in May (50.0%); and detritus was more abundant in September (30.6%) on par with Vegetable remains.
Vegetable remains (33.5%) was the most food group with greatest weight composition, followed by detritus (32.8%), fishes (22.3%), insects (8.3%) and other types (3.1%); being the main food in four months of the year: July (58.7%), October (59.5%), November (86.0%) and December (44.9%), while detritus was greater in January, June, August and September (Figure 2).
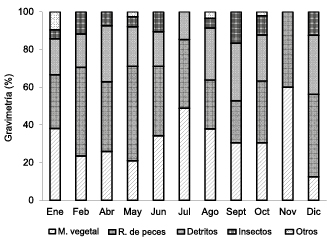
Figure 2. Composition by weight of prey.
The gut length-total length relationship (LI-LT) is estimated at 1.1, which places the species as omnivorous according to the scale proposed by Brusle (11). The correlation between the size of the gut and fish size (r = 0.53) is significant at 95% confidence according to the critical values for the correlation coefficients.
Analyzing food preferences in relation to size, in only three of the intervals (8.5-9.5, 9.5-10.5 and 10.5-11.5 cm LT) consumption of the five food groups (Vegetable remains, fishes, detritus, insects and other types) was found, while at intervals (7.5-8.5, 11.5-12.5 and 12.5-13.5) other groups were not observed and in the (13.5-14.5) range Vegetable remains was not found.
DISCUSSION
The emptiness coefficient estimated in this study (39.8%) is higher than reported for yellow Mojarra Caquetaia kraussii (29.2%), from the Cichlidae family in the Urrá dam (12), and which is high for a species that can be considered omnivorous due to the wide trophic spectrum presented. However, this ratio is within the range reported for other cichlids in different areas of South America such as in the Rodo Lake (Uruguay), where Cichlasoma facetum and Gymnogeophagus rhabdotus reached 35.0 and 49.0%, respectively (13).
As for the occurrence of different food groups, similarity was found in the variety but not to the extent shown in this work. Atencio-Garcia et al (12) observed that Caquetaia kraussii fish showed the highest occurrence (87.1%) while Vegetable remains, other types and insects barely reached 11.3, 6.5 and 1.6%, respectively. In Venezuela, the most frequent groups for A. pulcher and A. coerulopunctatus were Vegetable remains (94.0 and 95.0%), detritus (90.0 and 64.0%), insect larvae (72.0 and 73.0%), Coleoptera (26.2 and 32.3%) and Copepoda (21.2 and 25.6%) (14). While, the occurrence of major food groups in Geophagus brasiliensis (Cichlidae) in the Capivari Reservoir in Brazil was fruits and seeds (22.7%), detritus (17.9%), fishes (10.7%), Cladocera (7.0%), Copepoda (6.1%), Trichoptera (3.9%) and Chironomidae (3.9%) (15).
The abundance of prey found in this work is very different to that reported by other authors for the Cichlidae family in other geographical areas of Colombia and the rest of South America. Fish (83.3%), insects (1.4%), Vegetable remains (9.7%) and others (5.6%) were the largest groups in the diet of Caquetaia kraussii (12). As for composition by weight, fish (95.3%), insects (1.1%), Vegetable remains (3.5%) and others (0.1%) were the most important groups in the diet of Caquetaia kraussii (12). Here similarity was not observed between the proportion of trophic groups in the diets of different species, as happened with the frequency of occurrence and numerical frequency.
According to the data obtained in this study, the relative importance index indicates that Vegetables rest (21.4%), rest of fishes (14.0%) and detritus (14.3%) have secondary relative importance as food groups, while insects (1.2%) and other types (0.1%) are circumstantial or incidental prey and have low relative importance..
Analyzing the different analysis methods of stomach contents in this work, frequency of occurrence, numerical frequency, gravimetry, as well as the relative importance index (Table 1) shows that the food groups most consumed by Cocobolo are vegetable remains, rest of fishes and detritus, with an omnivorous diet, preferring fishes and vegetable remains.
Table 1. Annual values of frequency of occurrence (O), numeric frequency (FN), Gravimetry (G) and Index of relative importance of prey in the stomach of Cocobolo in Ciénaga Grande de Lorica.
Rojas et al (14) reported an omnivorous diet both for the species under study as well as A. coeruleopunctatus due to consumption of different resources such as phytoplankton, zooplankton, fish, Crustacea, insects (Hemiptera, Odonata and Ephemeroptera) and seeds. Therefore, taking into account issues raised by different authors, agreement is reached about eating habits but not concerning the main feed (12, 14-15).
It is observed that in low and rising waters, early and mid-year, the most consumed prey was fish, followed by vegetable remains and detritus, respectively. In high and falling waters, third and fourth quarters of the year, vegetable remains and fish were the most consumed trophic groups (Figure 3). Flexible eating habits are an adaptive feature of animal behavior when the natural environment varies spatially or temporarily, and fish respond to low food availability by altering their behavior (16). Most fish species seem to experience increased competition in shallow waters, which may be a consequence of reduced volumes of water or because flood areas, which provide food and cover in high waters, are not accessible (17), which leads many species to change their feeding preferences during the water cycle (18), as observed in this study.
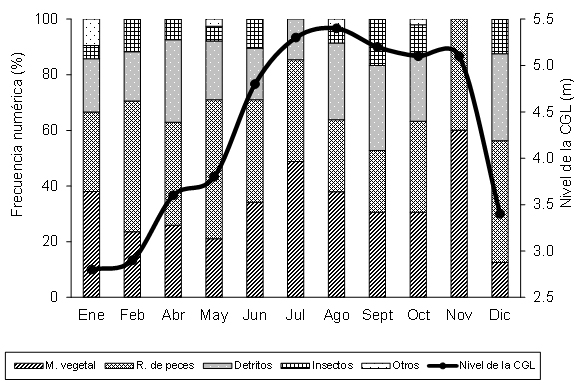
Figure 3. Feeding preferences of Cocobolo and the water cycle in Ciénaga Grande de Lorica.
The results of this study suggest that the Cocobolo has omnivorous feeding habits and a tendency to consume vegetable material.
Acknowledgments
To fishermen and fish traders in Ciénaga Grande de Lorica, lower basin of the Sinú River, and the University of Córdoba for funding received.
REFERENCES
1. Musilová Z, Říčan O, Novák J. 2009. Phylogeny of the Neotropical cichlid fish tribe Cichlasomatini (Teleostei: Cichlidae) based on morphological and molecular data, with the description of a new genus. J Zool Syst Evol Res 2009; 47(3):234-247.
2. Olaya-Nieto CW, Arellano Padilla JJ, Martínez González AL. Hábitos alimentarios del Barbul de piedra (Ariopsis sp.) en el río Sinú, Colombia. Acta biol Colomb 2012a; 17(1):117-128.
3. Olaya-Nieto CW, Pacheco-Orozco L, Ochoa-Arteaga J. Hábitos alimenticios del Liso (Rhamdia quelen Quoy & Gaimard, 1824) en el río Sinú, Colombia. Rev MVZ Córdoba 2012b; 17(3):3217-3223.
4. Maldonado-Ocampo JA, Ortega-Lara A, Usma-Oviedo JS, Galvis G, Villa-Navarro FA, Vásquez L, et al. Peces de los Andes de Colombia. Bogotá D.C.: Instituto de Investigación de Recursos Biológicos “Alexander Von Humboldt”; 2005.
5. Olaya-Nieto CW, Bautista-Blanco AL, Pérez-Pisciotti M. Biología reproductiva del Cocobolo (Andinoacara pulcher Musilová et al., 2009) (Pisces: Cichlidae) en la ciénaga Grande de Lorica (Córdoba), Colombia. Actual Biol 2010; 32(92):65-73.
6. IGAC, Instituto Geográfico Agustín Codazzi. Estudio General de Suelos y Zonificación de Tierras: departamento de Córdoba, escala 1:100.000. Bogotá: Imprenta Nacional de Colombia; 2009.
7. Laevastu T. Manual de métodos de biología pesquera. Zaragoza: Editorial Acribia; 1980.
8. Windell JT. Food analysis and rate of digestion. In: Ricker WE. (ed.). Methods for assessment of fish production in fresh waters. 2nd edition. Oxford: Blackwell Scientific Publications; 1971.
9. Windell JT, Bowen SH. Methods for study of fish diets based on analysis of stomach contents. In: Bagenal T. (ed.). Methods for assessment of fish production in fresh waters. 3rd edition. Oxford: Blackwell Scientific Publications; 1978.
10. Yáñez-Arancibia A, Curiel-Gómez J, Leyton V. Prospección biológica y ecología del bagre marino Galeichthys caerulescens (Günther) en el sistema lagunar costero de Guerrero, México (Pisces: Ariidae). An Centro Cienc del Mar y Limnol Univ Nal Autón México 1976; 3(1):125-180.
11. Brusle J. Food and feeding in grey mullet. In: Oren OH. (ed.). Aquaculture of grey mullet. Cambridge: Cambridge Univ Press; 1981.
12. Atencio-García VJ, Kerguelén-Durango E, Cura E, Rosado R, Vallejo A, Valderrama M. Régimen alimentario de siete especies ícticas en el embalse de la Hidroeléctrica Urrá (Córdoba, Colombia). Rev MVZ Córdoba 2005; 10(2):614-622.
13. Yafe A, Loureiro M, Scasso F, Quintans F. Feeding of two Cichlidae species (Perciformes) in an hypertrophic urban lake. Iheringia Sér Zool 2002; 92(4):73-79.
14. Rojas JE, Soca LA, García GI. Contenido del tracto digestivo de 4 especies de peces autóctonos y sus implicaciones como biorreguladores de larvas de mosquitos en Venezuela. Rev Cubana Med Trop 2005; 57(3):9-12.
15. Abelha MCF, Goulart E. Oportunismo trófico de Geophagus brasiliensis (Quoy & Gaimard, 1824) (Osteichthyes, Cichlidae) no reservatório de Capivari, Estado do Paraná, Brasil. Acta Scientiarum Biological Sciences 2004; 26(1):37-45.
16. Balassa GC, Fugi R, Hahn NS, Galina AB. Dieta de espécies de Anostomidae (Teleostei, Characiformes) na área de influencia do reservatório de Manso, Mato Grosso, Brasil. Iheringia Sér Zool 2004; 94(1):77-82.
17. Hawlitschek O, Yamamoto KC, Carvalho-Neto, FGMR. Diet composition of fish assemblage of Lake Tupe, Amazonas, Brazil. Rev Colombiana cienc Anim 2013; 5(2):313-326.
18. Mérona B, Rankin-de-Mérona. J. Food resource partitioning in a fish community of the Central Amazonian Floodplain. Neotrop Ichthyology 2004; 2(2):75-84.