ORIGINAL
Effect of different levels of L-carnitine and lysine-methionine on broiler blood parameters
Efecto de suplementación con L-carnitina y lisina-metionina sobre parámetros de sangre de pollos de engorde
Babak Hosseintabar,1 M.Sc, Mohammad Dadashbeiki,2 DVM, Mehrdad Bouyeh,1 Ph.D, Alireza Seidavi,1* Ph.D, René van den Hoven,3 Ph.D, Sandra Gamboa,4,5, Ph.D.
1Islamic Azad University, Rasht Branch, Department of Animal Science, Rasht, Iran,
2Islamic Azad University, Rasht Branch, Department of Veterinary Science, Rasht, Iran,
3Vetmeduni Wien, Vienna, Austria,
4Polytechnic Institute, Agricultural School, Department of Zootechnic Sciences, Animal Reproduction Laboratory, Coimbra, Portugal,
5CERNAS- Centre for Studies on Natural Resources, Environment and Society, Polytechnic Institute, Coimbra, Portugal.
*Correspondence: alirezaseidavi@iaurasht.ac.ir
Received: June 2014; Accepted: December 2014.
ABSTRACT
Objective. In the present study a completely randomized 3x3 factorial design was used to analyze the effects of different levels of L-Carnitine, lysine(Lys) and methionine (Met) on the blood concentrations of energy, protein and lipid metabolites of male broiler chickens. Materials and methods. A total of 270 newly hatched male broiler chickens (Ross 308) were randomly assigned to 9 groups (ten broilers per replicate and three replicates per treatment). The control group was fed a basal diet, whereas the treatment groups were fed basal diets supplemented with L-Carnitine (0 mg/kg, 75 mg/kg and 150 mg/kg) and lysine-methionine (0, 15 and 30%) for 42 days. On day 42, one bird was randomly chosen per replication, a blood sample was taken and the blood concentrations of glucose (GLU), uric acid (UAc), triglyceride (TG), VLDL, HDL, LDL, total protein (TP), albumin (Alb) and total cholesterol (TC) were analyzed. Results. Dietary L-carnitine supplementation had a significant effect (p<0.05) on uric acid (UAc), HDL, LDL, and total cholesterol (TC). The birds feed L-carnitine plus Lys and Met presented the highest plasmatic UAc level and the lowest plasmatic TC and LDL level. Moreover, L-carnitine significantly reduced total cholesterol (TC) when compared with both the control group and the birds feed Lys and Met without L-carnitine. Conclusions. A diet with 150 mg/kg L-carnitine plus 15% Lys and Met seems to be enough to sustain low plasmatic TC, LDL and HDL concentrations on male broiler.
Key words: amino acids, broiler chicken,lipid profile, serum metabolites, supplementation (Source: CAB).
RESUMEN
Objetivo. Se realizó un estudio para determinar el efecto de la suplementación deL-carnitina, lisina (Lys) y metionina (Met) sobre los metabolitos sanguíneos de pollos de engorde. Materiales y métodos. Se utilizaran 270 pollos de la línea Ross 308 de un día de edad y se dividieron en 9 tratamientos en un diseño al azar con arreglo factorial 3x3: tres niveles de L-carnitina (0 mg/kg, 75 mg/kg y 150 mg/kg) y tres de lisina-metionina (0, 15 y 30%) durante 42 días. Cada tratamiento constó de 3 repeticiones con 10 pollos por repetición. El día 42 de edad, se tomaron muestras de sangre de tres aves por tratamiento para cuantificar niveles séricos de glucosa (GLU), ácido úrico (UAc), triglicéridos (TG), VLDL, HDL, LDL, proteínas totales (PT), albúmina (Alb) y colesterol total (TC). Resultados. La suplementación de L-carnitina en la dieta tuvo un efecto significativo (p<0.05) en los niveles de ácido úrico en suero (UAC), HDL, LDL y colesterol total (CT). Las aves alimentadas con L-carnitina más Lys y Met mostraron niveles séricos más altos de UAc y menor TCy LDL. Por otra parte, la L-carnitina redujó significativamente el colesterol total (CT), cuando se comparó con el grupo control y con los pollos alimentados con Lys y Met, sin L-carnitina. Conclusiones. Una dieta con 150 mg/kg de L-carnitina y 15% Lys y Met parece ser suficiente para mantener bajas concentraciones plasmáticas de TC, LDL y HDL en pollos de engorde.
Palabras clave: Aminoácidos, metabolitos séricos, perfil lipídico, pollos, suplementación (Fuente: CAB).
INTRODUCTION
The increase in world population and the increase in household purchasing power are the factors that determine the future consumption of meat. Per capita, the global meat consumption is around 14 kg per year and the trend is upwards. The poultry belongs to the types of meat which can be produced relatively efficiently with low environmental impact (1). Moreover, the poultry meat has maintained a higher value compared to other species for several reasons including nutritional aspects as low-fat with high unsaturation degree of fatty acids and low cholesterol level.
The growing demand for poultry meat has resulted in pressure on breeders to increase the growth rate of birds, feed efficiency, size of breast muscle and reduction in abdominal fatness (2). Therefore, research is being directed to further improve techniques of poultry meat production. The improvement in carcass composition with additives has become a focus on nutrition research. The addition of amino acids and metabolic intermediates to diets may lower the abdominal fat deposition in poultry. L-carnitine, the biological active form of carnitine, is a quaternary ammonium compound synthesized in the liver, kidney and brain (3), from the essential amino acids lysine (Lys) and methionine (Met).
The effects of dietary L-carnitine, Lys and Met supplementations on the growth performance and body composition of broiler chickens are still poorly understood. Some authors reported that abdominal fat deposition in broilers is reduced by L-carnitine supplementation without significant effect on daily gain or feed conversion (4), while others found no impact of dietary L-carnitine supplementation on abdominal fat composition (5). However, Bouyeh and Gevorgyan (6) and Celik et al (7) showed that growth performance of broilers was improved by L-carnitine supplementation. Regarding Lys and Met supplementation in excess of NRC recommendations, Mukhtar et al (8) found a significant improvement in feed intake, average body weight gain and feed conversion ratio, while Si et al (9) concluded that it should not be elevate the level of Met if Lys is in excess of its minimum needs.
Corduk et al (10) have demonstrated that addition of L-carnitine to diet did not significantly affect body weight gain, feed intake, feed conversion ratio and blood parameters in broiler chickens. However, Parizadian et al (11) found that supplementing diet with L-carnitine will reduce blood triglyceride, cholesterol and improve egg quality in laying Japanese quail. Therefore, lower body fat deposition may be attributed to increased fat catabolism or diminished endogenous fatty acid synthesis or both processes (12).
The bioavailability of L-carnitine varies due to dietary composition but since it can be biosynthesized endogenously from methionine and lysine, these two amino acids are usually the more important limiting amino acids in poultry nutrition. Methionine and lysine are frequently supplemented in the formulated diets. Previous studies showed that methionine supplement during the early stages of chicken growth causes significant improvement in hematocrit, red blood cell count and related parameters (13,14).
Theoretically, supplementing the broiler diet with an adequate content of L-carnitine would facilitate the β-oxidation of fatty acids, and decrease esterification reactions and triacylglycerol storage in the adipose tissue (12). As blood biochemistry may monitor the quality of nutrition and health of birds (13,15), we have quantified serum markers of lipid and protein metabolism on 42 day older male broiler chickens feed extra levels of dietary Lys-Met with and without L-carnitine.
The objetive of this study was to evaluate the effects of different levels of L-carnitine, Lys and Met on the blood concentrations of energy proteins and lipid metabolites of male broiler chickens.
MATERIALS AND METHODS
Study site. This study was developed in the poultry farm facilities of the Agriculture Faculty of Islamic Azad University, in Rasht, Iran. It is situated 7 m below sea level and located at latitude 37°15’ N and longitude 49°36’ E.
Facilities. The facilities (20x40 meters) included, other than the main compartment for chicken, a storage room, where the ingredients are grinded and mixed. Six ventilators were used to maintain an air discharge power of 1400 cubic meters per hour. During the experiments, room temperature was maintained applied according to the instructions of the Ross 208 strain rearing manual. The temperature was adjusted to approximately 33°C on the day before day 1. The birds were housed in the main compartment, in 27 pens with 1.0 x 2 m. Prior to the experiment the hall and all equipment was carefully cleaned and disinfected according to general bio-security procedures for broiler production. Temperature, light, ventilation, drinkers and feeders were all managed according to the Ross 208 manual for production and were equal for all treatments.
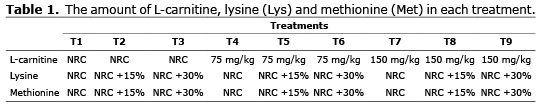
Animals and experimental design. A total of 270 newly hatched male Ross 308 broilers were used for the experiment. The birds had been sourced from the Ross hatchery in Iran. After sexing, animals were randomly allocated to the experimental units. A total of 9 treatments groups were formed (Table 1). Each group had 3 replicates with 10 birds per replicate. Animals were reared for 42 days:birds received starter diet from 1-21 d, grower diet from 22-35 d and finisher diet from 36-42 d of age, respectively. The L-carnitine, Lys and Met levels were according to NRC (16) recommendations for starter (1.1Lys and 0.9 Met), grower (1.0 Lys and 0.72 Met) and finisher (0.8 Lys and 0.9 Met) feed.
The control group ration was the basis of all other diets (Table 2). In the experimental diets, extra L-carnitine, Lys and Met were added in excess of NRC (16) recommendations according to table 2.
Sampling and blood parameters. At the end of the experiment, at42 days-of-age, one bird per group was randomly selected for blood collection. Blood samples (1 ml/bird) were collected using a 2 ml syringe and left to stand at 30°C to allow clotting and clot retraction. These rum that remained after clotting was centrifuged at 3000 rpm for 10 minutes at room temperature. Blood parameters analyzed in serum were glucose (GLU), uric acid (UAc), total triglycerides (TG), total cholesterol (TC), total protein (TP), albumin (Alb), high density lipoprotein (HDL), low density lipoprotein (LDL)and very low density lipoprotein (VLDL).The concentrations for these parameters were determined by routine methods using commercial laboratory kits (Pars Azmoon Co., Tehran, Iran), according to the manufacturer’s instructions. GLU was measured with an enzymatic, colorimetric glucose oxidase-peroxidase (GOD-POD) method, while TC, TG, HDL, LDL and VLDL were determined by enzymatic CHOD-PAP assays. Alb was determined based on the bromocresol green method, whilst the UAc was determined by enzymatic methods using the uricase-TOOS method and TP was assayed by the Biuret method.
Statistical analysis. For the 3x3 factorial design the linear model Yijk=µ+αj+βk+(αβ)jk+εijk was applied, in which the value of each observation (Y) relies on the population effects average (µ) plus the effect of factor A (αj, L-carnitine level), the effect of factor B (βk, methionine-lysine level) and their interaction [(αβ)jk], and the experimental error (ε). A general mixed model was chosen as most robust statistical test that respected the repeated measures. The replicate was the experimental unit (17).
RESULTS
The results for the biochemical parameters concentration at day 42 in broilers plasma according to feed treatments are summarized in table 3.
At day 42, no significant changes were found in the mean blood parameters concentrations between birds submitted to extra Lys-Met supplementation without extra L-carnitine (T2 and T3) and the control group (T1). However, extra Lys-Met supplementation alone showed a discreet decay in plasmatic total cholesterol (TC) and low density lipoprotein (LDL) when compared with the control diet (T1) (Table 3). No main effects were evident for Lys plus Met supplementation across treatments (Table 4).
The supplementation of the diet with L-carnitine and Lys plus Met had no effect (p>0.05) in plasmatic glucose (GLU), triglyceride (TG), total protein (TP), albumin (Alb) and low density lipoprotein (VLDL). In contrary, the effect of treatments on plasmatic uric acid (UAc), cholesterol (TC), low density lipoprotein (LDL) and high-density lipoprotein (HDL) levels was significant (p<0.05) (Table 3). Main effects were evident for L-carnitine supplementation across treatments for plasmatic uric acid (UAc), cholesterol (TC) and low density lipoprotein (LDL),(Table 5).
The lowest concentrations in plasmatic UAc and the highest concentrations in plasmatic TC and LDL were observed in treatments where no L-carnitine was added (T2 and T3), whereas the highest concentrations in plasmatic UAc and the lowest concentrations in plasmatic TC and LDL were seen in the groups supplemented with 150 mg/kg L-carnitine (T7, T8 and T9).
Concerning plasmatic uric acid (UAc) concentration, a significant difference (p<0.05) was found between birds feed 150 mg/kg L-carnitine plus 30% Lys and Met (T9) and the control group. A statistical difference was also found for high density lipoproteins (HDL, p<0.01) concentration between the control group and the birds feed 150 mg/kg L-carnitine plus 15% Lys and Met (T8) (Table 3).
The total cholesterol (TC) and low density lipoprotein (LDL) were significantly reduced (p<0.05) when L-carnitine was added, with or without Lys and Met. In groups fed 150 mg/kg L-carnitine plus Lys and Met (T8 and T9), plasmatic cholesterol (TC) concentration was significant lower (p<0.05) than in groups without L-carnitine supplementation (T1, T2 and T3) and in birds feed 75mg/kg L-carnitine without Lys and Met (T4). As to, plasmatic LDL concentration in groups submitted to 75 mg/kg L-carnitine plus 30% Lys and Met (T6) and 150 mg/kg L-carnitine without and with Lys and Met (T7, T8 and T9), differed significantly (p<0.01) from groups without L-carnitine supplementation (T1, T2 and T3) and in birds feed 75mg/kg L-carnitine without and with 15% Lys and Met (T4 and T5) (Table 3).
DISCUSSION
An exact characterization of the metabolites’ profile in the birds of the various treatment groups is not possible since only one blood sample was collected at day 42. However, using some markers of energy, protein and lipid metabolism such as glucose, cholesterol, triglyceride, uric acid, albumin and total protein, the obvious changes in carbohydrate, fat and protein metabolism are likely detected. The interpretation of results of the blood biochemistry must be combined with total growth, meat weight, fat weight and meat quality in order to be useful for designing new diets. Nevertheless, serum triglyceride (TG), total cholesterol (TC), LDL, and HDL are crucial biochemical parameters reflecting the status of lipid metabolism.
In this study, the Lys-Met supplementation per si had no effect on blood parameters at day 42 but in combination with extra L-carnitine significant differences were observed among groups for the uric acid (UAc), total cholesterol (TC), LDL and HDL concentrations in broiler plasma at day 42.
In broilers, uric acid (UAc) is produced as the main end product of nitrogen metabolism. The dietary protein level markedly affects uric acid production. Our results showed that UAc increase as dietary L-carnitine, Lys and Met intake increases. However, inconsistent results have been reported in the literature regarding the use of these amino acids in poultry diets. Donsbough et al (18) described a decrease in UAc when dietary Lys was increased in the diet while Xie et al (19) described a decrease in serum UAc concentrations followed by an increase as the dietary Met level increased.
In our study, concentration of protein and albumin were not affected by different levels of L-carnitine and Lys-Met. L-carnitine induced significant increases of serum albumin (Alb) concentrations in dietary energy depleted birds without affected total protein (20). Most studies on Met could not prove an effect on serum total protein and albumin in broilers (13,21).
The plasmatic glucose (GLU) and free fatty acids concentrations are good indicators of the energetic status. The dynamic interactions between these two major energy substrate pools, without hormonal mediation seems to indicate that the utilization of one nutrient (e.g. glucose) directly inhibits the use of the other (in this case fatty acids) in accordance with Arslan et al (22).
Mitochondrial fatty acid β-oxidation is an important system by which fatty acids are broken down by various tissues to produce energy. In this system, L-carnitine plays an important role by facilitating the transport of fatty acids from cytosol across the inner mitochondrial membrane.
In our study, the concentration of glucose (GLU) and triglyceride (TG) was not affected by different levels of L-carnitine and Lys-Met in the diet. These results are in accordance with the study of Taraz and Dastar (14) since they showed that the concentration of GLU and TG was not affected by L-carnitine and Lys-Met supplementation. As well, Arslan et al (22) could neither find an effect of L-carnitine on plasmatic GLU concentrations. Conflicting results were reported by Bouyeh and Gevorgyan (6). However, our results are against the result of Xu et al (4). These authors described that L-carnitine causes the decrease of the plasmatic concentration of triglyceride (TG). Moreover, they found that by adding 50, 75, or 100 mg/kgL-carnitine to diets the total activities of NADPH-generating enzymes (G-6-PD, MDH, and ICD) in subcutaneous fat were decreased (p<0.05) and the synthesis of fatty acid in subcutaneous fat would accordingly be decreased.
In the present study, the concentration of TC, LDL and HDL were affected by different levels of L-carnitine and Lys-Met, whereas VLDL plasma concentrations were not affected. Bouyeh and Gevorgyan (6) observed a decrease followed by an increase in plasma cholesterol in birds feed Lys and Met above NRC recommendations. It is possible that this was caused by increased cholesterol storage in muscles. Cholesterol is probably less actively stored and need a longer period of ingestion before significant increase or decrease may show up (6). In this study, the plasmatic total cholesterol (TC) and LDL were significantly reduced when L-carnitine was added, with or without Lys and Met. These results agree with those from Murali et al (23). However, the data by Taraz and Dastar (14), Adabi et al (24) and Arslan et al (22) do not agree with our findings.
The very low density lipoprotein (VLDL) and LDL are closely related to one another. VLDL is formed in the liver and is converted to LDL in muscle and adipose tissues by losing fatty acids. The LDL thus produced supply the tissues with cholesterol. The TG accumulation in adipocytes depends mainly on the availability of plasma VLDL and genetically fat chickens presented high levels of VLDL. In this study, despite no differences were found between treatments, the plasmatic VLDL concentration were low.
Taken together, our data show that a diet containing 150 mg/kg L-carnitine with 15% Lys and 15% Met seems to be enough to sustain low levels of TC, LDL and HDL. Therefore, L-carnitine may decrease body fat content in broiler carcasses by decreasing the deposition of fatty acids in extra hepatic tissues, above all the adipose and muscle tissue.
Acknowledgments
Financial support by Rasht Branch, Islamic Azad University, grant number 4.5830 is gratefully acknowledged.
REFERENCES
1. Hamerschlag K. Meat Eaters Guide to Climate Change + Health. Environmental Working Group. Meat Eaters Guide: Report 2011. [cited 2014 Mai 22] Avaible from:http://static.ewg.org/reports/2011/meateaters/pdf/report_ewg_meat_eaters_guide_to_health_and_climate_2011.pdf
2. Petracci M, Mudalal S, Bonfiglio A, Cavani C.Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poult Sci 2013; 92(6):1670-1675.
3. Cave MC, Hurt RT, Frazier TH, Matheson PJ, Garrison RN, McClain CJ et al. Obesity, inflammation, and the potential application of pharmaconutrition. Nutr Clin Pract 2008;23:16-34.
4. Xu ZR, Wang MQ. Effects of L-carnitine on growth performance, carcass composition, and metabolism of lipids in male broiler. Poult Sci 2003;82:408-413.
5. Kidd MT, Gilbert J, Corzo A, Page C, Virden WS, Woodworth JC. Dietary L-carnitine influences broiler thigh yield. Asian-Aust J Anim Sci 2009; 22(5):681-685.
6. Bouyeh M, Gevorgyan O. Influence of excess lysine and methionine on cholesterol fat and performance of broiler chicks. J Anim Vet Adv 2011; 10(12):1546-1550.
7. Celik L, Ozturkcan O, InalL TC, Canacankatan N, Kayrin L. Effects of L-carnitine and niacin supplied by drinking water on fattening performance, carcass quality and plasma L-carnitine concentration of broiler chicks. Arch Anim Nutr 2003;57(2):127-136.
8. Mukhtar MA, Mohammed KA, Musa MH.Replacement value of lysine and methionine for super concentrate in broiler chick’s yield and quality. J Sc Tech 2010; 11(2):27-29.
9. Si J, Kersey JH, Fritts CA, Waldroup PW. An evaluation of the interaction of lysine and methionine in diets for growing broilers. Int J Poult Sci 2004; 3(1):51-60.
10. Corduk M, Ceylan N, Ildiz F. Effects of dietary energy density and L-carnitine supplementation on growth performance, carcass traits and blood parameters of broiler chickens. South African J Anim Sci 2007; 37(2):65-73.
11. Parizadian B, Ahangari YJ, Shargh MS, Sardarzadeh A. Effects of Different levels of L-carnitine supplementation on egg quality and blood parameters of laying japanese quail. Intern J Poult Sci 2011; 10(8):621-625.
12. Arslan C. L-carnitine and its use as a feed additive in poultry feeding a review. Revue Méd Vét2006; 157(3):134-142.
13. Al-Mayah AAS. Immune response of broiler chicks to DL-methionine supplementation at different ages. Int J Poult Sci 2006; 5:169-172.
14. Taraz Z, Dastar A. Effect of L-carnitine supplementing diets with different levels of protein on performance and blood parameters in broiler chickens. J Agric Sci Nat Res2008; 15(5):114-122.
15. Etim NAN, Akpabio U, Okpongete RO, Offiong EEA. Do Diets Affect Haematological Parameters of Poultry? British J Appl Sci & Techn 2014; 4(13):1952-1965.
16. National Research Council (NRC). Nutrient Requirements of Poultry: Ninth Revised Edition. Washington, DC: The National Academies Press, 1994.
17. SAS, 2000. Statistical Analysis System. SAS User Guide: Release 9.2. SAS Institute INC, Cary NC, USA, 2000.
18. Donsbough AL, Powell S, Waguespack A, Bidner TD, Southern LL. Uric acid, urea, and ammonia concentrations in serum and uric acid concentration in excreta as indicators of amino acid utilization in diets for broilers. Poult Sci 2010; 89:287-94.
19. Xie M, Hou SS, Huang W, Zhao L, Yu JY, Li WY et al. Interrelationship between methionine and cysteine of early peking ducklings. Poult Sci 2004; 83:1703-1708.
20. Çakir S, Yalçin S Effects of L-carnitine supplementation in diets with low or normal energy level on growth performance and carcass traits in broilers. Revue Méd Vét 2007; 158(6):291-296.
21. Chattopadhyay K, Mondal MK, Roy B. Comparative efficacy of DL-methionine and herbal methionine on performance of broiler chicken. Int J Poult Sci 2006; 5:1034-1039.
22. Arslan C, Citil M, Saatci M. Effects of L-carnitine administration on growth performance, carcass traits, serum lipids and abdominal fatty acid compositions of geese. Rev Med Vet 2004: 155(6):315-320.
23. Murali P, George SK, Mercy AD, Dipu MT, Narayanankutty K. Effects of L-carnitine supplementation on serum lipid profile of broiler chicken fed with animal fat. Int J Agric and Food Sci Technol 2013; 4(8):773-774.
24. Adabi ShG, Moghaddam Gh, Taghizadeh A, Nematollahi A, Farahvash T. Effect of L-carnitine and vegetable fat on broiler breeder fertility, hatchability, egg yolk and serum cholesterol and triglyceride. Int Poult Sci 2006; 5(10):970-974.