ORIGINAL
Subclinical laminitis and its association with pO2 and faecal alterations: Isikli, Aydin experience
Laminitis subclínica y su asociación con pO2 y alteraciones fecales: experiencia Isikli, Aydin
Ibrahim Akin,1* Ph.D, Deniz Alic Ural,2 Ph.D, Mehmet Gultekin,3 Ph.D, Kerem Ural,3 Ph.D.
Adnan Menderes University, Faculty of Veterinary, 1Department of Surgery, 2Faculty Farm, 3Department of Internal Medicine, 09016, Isikli, Aydin-Turkey.
*Correspondence: ibraak@hotmail.com
Received: June 2014; Accepted: December 2014.
ABSTRACT
Objective. The aim of this field trial was to investigate the relationships among subclinical laminitis, hematological, ruminal and faecal alterations. Materials and Methods. To this extent dairy cows presenting subclinical laminitis (n=11) and to those of other healthy cows without laminitis (n=10) were enrolled and assigned into two groups. All animals were receiving the same daily ration formulated to contain 47% cornsilage and 18% hay, mainly. Effects of subclinical laminitis challenges on measurements of feces, and blood samples, were investigated to determine which of these measurements may aid in the diagnosis. pH changes in ruminal fluid collected via rumenocentesis were measured. Besides the following parameters were also measured; blood pH, faecal pH and faecal scoring. Blinded investigators performed the sample collection. Results. No statistical differences between the groups were detected for blood gas values studied regarding pCO2, HCO3, BE, indeed mean that pO2 values decreased statistically (p<0.05) and faecal pH was significantly decreased (p<0.05) in cows with subclinical laminitis in contrast to healthy controls. Conclusions. pO2 values and faecal pH may be valuable as indirect indicators of subclinical laminitis in cattle
Key words: Dairy cattle, laminitis, nutrition (Source: CAB).
RESUMEN
Objetivo. El objetivo de esta prueba de campo fue investigar las relaciones entre la laminitis subclínicay alteraciones hematológicas, ruminales y fecales. Materiales y métodos. Las vacas lecheras que presentaron laminitis subclínica (n=11) y las vacas sanas sin laminitis (n=10) fueron reclutadas y asignadas en dos grupos. Todos los animales recibieron la misma ración diaria que contenía 47% de ensilaje de maíz y 18% de heno, principalmente. Los efectos de la laminitis subclínica sobre las mediciones de las heces y muestras de sangre, fueron investigados para determinar cuál de estas mediciones pueden ayudar en el diagnóstico. Se midieron los cambios de pH en el fluido ruminal recogido a través rumenocentesis. Además, también se midieron los siguientes parámetros; pH de la sangre, el pH fecal y la puntuación fecal. La toma de las muestras se realizó a doble ciego. Resultados. No se detectaron diferencias significativas entre los grupos para los valores de los gases sanguíneos estudiados en relación con la pCO2, HCO3, BE; lo que significa que los valores de pO2 disminuyeron estadísticamente (p<0.05) y que el pH fecal se redujo significativamente (p<0.05) en las vacas con laminitis subclínica; en contraste con los controles sanos. Conclusiones. Los valores de pO2 y pH fecal pueden ser valiosos como indicadores indirectos de la laminitis subclínica en el ganado.
Palabras clave: Ganado de leche, laminitis, nutrición (Fuente: CAB).
INTRODUCTION
The economic significance and animal welfare consequences of lameness in dairy cattle are substantial (1). Subclinical laminitis (inflammation of the corium within the hoof) is a well recognized trigger condition for many of disorders in the hoof, and is considered by predisposing factor in lameness in dairy cattle (2,3). Therefore, it has been considered the main factor to iniating lameness following lactations in dairy cows (4).
Lameness is one of the major cow health concerns regarding dairy industry (5-7). Physical etiology of lameness, are discussed in detail (3,8,9), indeed nutrition is frequently dictated as a major cause of lameness. Despite what has been discussed, the significant relationship regarding lameness and dietary composition is comparatively poorly described. Contrarily, ruminal functioning is almost suggested as the predisposing factor, even if lameness is attributable to nutrition (10). On the basis of previous works lacking detailed information, the present authors examined ruminal pH, blood pH, faecal pH and blood gas during subclinical laminitis in dairy cows with the aim of identifying any relationships among laminitis and those aforementioned physiological parameters in association to a suitable nutrition.
MATERIAL AND METHODS
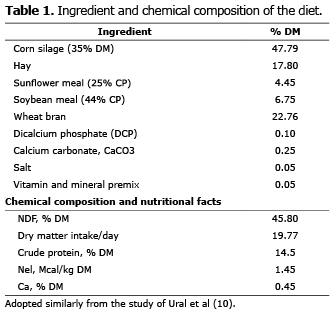
Study site and geographical location. The present retrospective study was performed in a private dairy farm located at Isikli, Aydin province of Turkey. Aydin city is located at the Eagean region of Turkey. The dairy farm consisted 500 dairy cattle. The latter city has warm climatic conditions in general, which is quite suitable for dairy farming. The diet involved for the dairy cattle in the present study was shown in table 1.
Inclusion criteria and sampling. Cows enrolled in the present study were examined in the hoof trimming chute in an attempt to evaluate the presence of the clinical findings (involving yellowish discoloration and waxy hoof, heel erosion, sole haemorrage, white line separation, abnormal hoof grown), of subclinical laminitis (11). Cows, diagnosed within subclinical laminitis had two or more mentioned laminitis-like lesions of the subclinical laminitis, and were enrolled within the study as subclinical laminitis group. Healthy group did not present mentioned lesions and regular hoof shape was detected.
Regarding time of sampling for collection of ruminocentesis and other relevant parameters, samples were collected at a time when rumen pH is likely to be near the lowest point of the day, as reported previously (12). As the ration was fed as a total mixed ration (TMR), the samples were withdrawn at 4 hours after the cows get access to the fresh ration. All samples were collected once throughout the study period.
Laboratory measurements
Rumen pH measurement. The pH of the rumen fluid samples collected at 15.00 of day time, as reported previously (13), at optimal recommended timing for ruminal pH measuremets, 5 hours after forage and concentrated fed separately were determined immediately after collection with an electronic caliber Accumet Basic 15 pH meter. The pH data were summarized as minimum, maximum and average pH.
Faecal sampling and analysis. Faecal samples, were collected after restraining the animals and disinfection of the perianal area. Each individual sample was photographed, and then was thoroughly mixed. A subsample of 10 g was mixed into distilled water of 10 ml. This was followed by the faecal pH measurement using the same caliber, used for the rumen fluid pH measurement.
Blood sampling and analysis. Whole blood withdrawn into vacutainer tubes with K3EDTA, was analyzed at the Veterinary Faculty, Central Laboratory located in Adnan Menderes University, Aydin (data not shown). Heparinized whole blood was also analyzed for pH, pCO2, pO2, Na+, K+, and Ca2+ using a Irma Trupoint blood gas analyzer.
Statistical analysis. Normal distributed data were analyzed by independent samples t-test to compare means of two groups. Faecal scores were analyzed by Mann-Whitney U test. p≤0.05 were considered to be significant. The results were presented as the means ± SE.
RESULTS
The average values of analyzed parameters and their standard error of the means were presented in table 2.
No statistical differences between the groups were detected for blood gas values studied regarding pCO2, HCO3, BE, indeed mean pO2 values decreased statistically (p<0.05) in cows with subclinical laminitis.
Ruminal fluid pH changes. Normal rumen pH condition was detected within the vast majority of the animal enrolled in group ScL, whereas only one cow with subclinical laminitis had a ruminal pH value of 5.79, in which is a critical value regarding subacute ruminal acidosis (SARA) (14-17).
Faecal alterations. Faecal pH and faecal scoring results were shown in table 2. Analysis deemed statistical significance for faecal pH values.
Subclinical laminitis signs. Relevant clinical findings were shown in figure 1.
DISCUSSION
Ruminal changes and its interpretation. In the present study Subacute Ruminal Acidosis (SARA) was not a contributing factor the relationship among physiological parameter detected and laminitis, as 1 cow was diagnosed with suspected SARA. Gozho et al (14) stated determining SARA by rumen pH between 5.2-5.6 for at least 3 hours continuously in a daily measurement via an indwelling pH catheter. For the entire context of the present study, ruminal acidosis was considered to occur when ruminal pH<5.8, and acute ruminal acidosis when pH<5.2, even if any case was observed. Indeed more severe alterations within the ruminal pH might be necessary to predict changes in ruminal epithelial functioning (16,17), the availability to use of pH 5.8 as a threshold bypass the efficacy of low pH on fiber digestion, as well as the probable risk regarding epithelial damage. In the present study solely 1 cow presented a ruminal pH value of 5.79, suggesting that other cows were not experiencing SARA, at least at the time of study enrollment. Therefore SARA was not accompanying to laminitis for the animals involved at the present study.
Faecal changes and its interpretation. The faeces of stock may provide significant knowledge regarding indirect proof of clinical and sub-clinical acidosis. Several factors involving parasites and diseases may result in scouring. Herds possessing large percentage of soft and un-formed faecal content, cows with a high prevalence scouring or faecal material within the perineum might be showing acidosis. This condition might be supported with other relevant indicators, including the prevalence of lameness, ration evaluation, rumen sampling, milk fat concentration and reduced chewing activity for a better and definitive diagnosis (9,13,15). As aforementioned above only 1 cow with a critical value for SARA was presenting scour, with a faecal score as 1. Interestingly the latter score did not have an effect on total clinical score in group ScL, resulting within no statistical significance between groups.
SARA alterations did not have influence on faecal pH (13,18). In a relatively new study it was suggested that there was differences in faecal pH between 09:00 and 15:00, indicating that the timing of the faecal pH analysis must be considered for evaluation of the faecal pH (13). In contrast Morgante et al (12) determined that cows with SARA [ruminal pH>5.8 (acidotic cows)] presented a faecal pH (6.50±0.04) lower than that from cows (6.65±0.03) without SARA (ruminal pH>5.8). Eastridge (19) stated that faecal pH might be reduced, even in case of excessive amounts of starch escape digestion, resulting in large elevation of hindgut fermentation. Relevant differences in the dietary concentrate contents among the present study and those of Li et al (13), Morgante et al (12) and Enemark et al (18) may be briefly explained within the discrepancy among the effects of SARA on faecal pH for those studies. Only one cow with laminitis was diagnosed within a critical value for SARA due to low ruminal pH, as 5.79, in the present study, whereas the rest of the animal did not present SARA.
Faecal pH was variable and did not correlate with the ration involved in the present study. Faecal pH showed differences among groups, in which cows with subclinic laminitis presented a significantly lower faecal pH. It has been well known that faecal pH monitoring is strongly dependent on duration between sampling and measuring, besides may be influenced by management factors, (cattle movement/feding, barn cleaning i.e.) (20). In the present study the duration between sampling and analysing was too short (within seconds), suggesting that time was excluded as a factor influencing analysis.
From another point of view as SARA alterations did not have influence on fecal pH (15, 18), whereas timing of the sampling had had disperancy on fecal pH as must be taken into consideration (15). Fecal pH is lowered when excessive amount of starch escape digestion from the rumen and intestines, resulting with an increased hindgut fermentation (19). It should not be unwise to draw a probable conclusion that lower fecal pH obtained among cows with subclinic laminitis may be due to increased hindgut fermentation. In aggreement within this opinion, available data from horse with laminitis suggested that metalloproteinase enzymes produced by Streptococcus bovis in the hindgut might be the existing cause for laminitis (21). Even if the latter enzymes are involved in dairy cattle laminitis, they may exist within hindgut origin. According to the latter hypothesis, laminitis may be a consequence of abnormal hindgut fermentation of carbohydrates instead of ruminal acidosis in cattle (22), as aforementioned above.
Significant blood gas alterations.
pO2 changes. Given the statistical changes in the present study, mean pO2 values decreased statistically (p>0.05) in cows with subclinical laminitis. This may be partly explained from the observations/conclusions arising in a prior study evaluating the blood gas changes in induced laminitis among horses (23). In an attempt to draw conclusion from the latter study one should precisely evaluate the results. During experimentally induced laminitis in horses there was a significant alteration in the median palmar venous pO2 and concurrent decline in the median palmar avO2, observed during the onset of lameness. According to the latter investigators, several factors might have influence on this.
There was no difference in the arterial oxygen pressure excluding out external conditions (i.e. mechanical or atmospheric influences). Besides no concurrent alteration was noticed in respiratory rate or frequency. Investigators suggested some probable explanations including: (i) changed blood flow over the capillary vessels (vasoconstriction/arteriovenous shunting); ii) lack of oxygen delivery to the tissues, iii) declined cellular utilization of oxygen, via altered tissue metabolism or any obstruction for cellular respiratory enzymes; iv) thickened or edematous vascular membrane resulting within a failure of oxygen to diffuse out of the capillaries to the tissues (23). pO2 decline detected in horse in the latter study involved digital arteries, however the blood samples and therefore the results of pO2 values were related to central blood. It must be mentioned that obtaining a venous sample is easier than an arterial sample. Venous blood may possess enough information for assisting in clinical decisions; besides the vast majority of blood gas values are similar in venous and arterial blood (24). In agreement within the latter authors, and as expected, the latter two conditions (i.e. iii and iv.) might play a pivotal role for pO2 alterations, during laminitis, as identified within the cows of the present study.
Oxygen supply into tissues is a life threatening requirement for survival. The pO2 is one of the major part of a physiological condition of an organ, resulting from the association among oxygen delivery and consumption. Tissue oxygenation is altered during pathological conditions (25), such as hepatic hypoxia (26) or laminitis in horses (23), that may result with decreased pO2, meant hypoxia a significant correlation was evident between hepatic tissue pO2 and arterial blood pO2 in hypoxic rabbits (26). Another study among horses with laminitis revealed that median palmar vein pO2 was increased in horses with laminitis.
On the other hand in dairy cows with subclinical laminitis no acid base imbalance were detected, whereas in that study in Portuguese language, solely abstract was available in English revealed that the results of pO2 was unknown (27). It should also be noted that hepatic hypoxia (26) might also play a role for significant pO2 changes detected. However the present authors could not draw this conclusion, as the limitation of the study was lack of hepatic enzyme analysis. Further analysis are warranted analyzing serum biochemical changes, evolving ALT, AST and other relevant hepatic enzyme changes. None of the cows in this study developed severe alterations in acid-base balance or hypothermia, which could have effected the availability of oxygen. It is possible to state that further research of this phenomenon may lead to the development of blood gas analysis, and probably pO2 as a presumptive diagnostic tool in cattle with subclinical laminitis.
Pain may accompany an autonomic response elevating adrenergic nerve activity and plasma catecholamine levels. As a result vasoconstriction of arterioles, reduced wound perfusion, and decreased tissue pO2 may occur (28). Therefore the present authors may possibly suggest that the other condition that might be resulting within the decreased pO2 in cattle with subclinical laminitis is that pain.
This study has demonstrated that there might be a correlation among subclinical laminitis and pO2 values. As aforementioned above and pO2 values might probably indicate the pathophysiological changes that occur as part of the pathogenesis of the condition. Besides faecal pH alternatively might be a supportive finding in cattle with subclinical laminitis, at least for the present cases observed in this study. Obtained data did not support that fecal pH may be an early indicator of laminitis, besides decreases in fecal pH both at onset and during disease condition, whereas it may be a helpful parameter in in cattle with subclinical laminitis.
REFERENCES
1. Richert RM, Cicconi KM, Gamroth MJ, Schukken YH, Stiglbauer KE, Ruegg PL. Perceptions and risk factors for lameness on organic and small conventional dairy farms. J Dairy Sci 2013; 96(8):5018–5026.
2. Sagliyan A, Gunay C, Han MC. Prevalence of lesions associated with subclinical laminitis in dairy cattle. Israel J Vet Med 2010; 65(1):27-33.
3. Lean IJ, Westwood CT, Golder HM, Vermunt JJ. Impact of nutrition on lameness and claw health in cattle. Livest Sci 2013; 156(1–3):71–87.
4. Pilachai R, Schonewille JTh, Thamrongyoswittayakul C, Aiumlamai S, Wachirapakorn C, Everts H, Hendriks WH. Diet factors and subclinical laminitis score in lactating cows of smallholder dairy farms in Thailand. Livest Sci 2013; 155(2–3):197–204.
5. Bell NJ, Bell MJ, Knowles TG, Whay HR, Main DJ, Webster AJF. The development, implementation and testing of a lameness control programme based on HACCP principles and designed for heifers on dairy farms. Vet J 2009; 180:178-188.
6. Harris DJ, Hibburt CD, Anderson GA, Younis PJ, Fitspatrick DH, Dunn AC, Parsons IW, McBeath NR. The incidence, cost and factors associated with foot lameness in dairy cattle in south-western Victoria. Aust Vet J 1988; 65:171-176.
7. Bicalho RC, Oikonomou G. Control and prevention of lameness associated with claw lesions in dairy cow. Livest Sci 2013; 156: 96–105.
8. Choquette-Levy L, Baril J, Levy M, St-Pierre H. A study of foot disease of dairy cattle in Quebec. Can Vet J 1985; 26: 278-281.
9. Enemark JMD. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): a review. Vet J 2008; 176:32–43.
10. Ural DA, Cengiz O, Ural K, Ozaydin S. Dietary clinoptilolite addition as a factor fort he improvement of milk yield in dairy cows. J Anim Vet Adv 2013; 12(1):85-87.
11. Greenough PR. Bovine laminitis and lameness: a hands-on approach. España: Elsevier; 2007.
12. Morgante M, Gianesella M, Casella S, Ravarotto L, Stelletta C, Giudice E. Blood gas analyses, ruminal and blood pH, urine and faecal pH in dairy cows during subacute ruminal acidosis. Comp Clin Pathol 2009; 18:229-232.
13. Li S, Khafipour E, Krause DO, Kroeker A, Rodriguez-Lecompte JC, Gozho GN, Plaizier JC. Effects of subacute ruminal acidosis challenges on fermentation and endotoxins in the rumen and hindgut of dairy cows. J Dairy Sci 2012; 95:294-303.
14. Gozho GN, Krause DO, Plaizier JC. Rumen lipopolysaccharide and inflammation during grain adaptation and subacute ruminal acidosis in steers. J Dairy Sci 2006; 89(11):4404-4413.
15. Li S, Gozho GN, Gakhar N, Khafipour E, Krause DO, Plaizier JC. Evaluation of diagnostic measures for subacute ruminal acidosis in dairy cows. Can J Anim Sci 2012; 92(3):353-364
16. Aschenbach JR, Gabel G. Effect and absorption of histamine in sheep rumen: Significance of acidotic epithelial damage. J Anim Sci 2000; 78:464–470.
17. Penner GB, Beauchemin KA. Variation among cows in their susceptibility to acidosis: Challenge or Opportunity? Adv Dairy Tech 2010; 22:173-187.
18. Enemark JMD, Jørgensen RJ, Kristensen NB. An evaluation of parameters for the detection of subclinical rumen acidosis in dairy herds. Vet Res Commun 2004; 28:687-709.
19. Eastridge ML. Major advances in applied dairy cattle nutrition. J. Dairy Sci 2006; 89:1311-1323.
20. Jacque K. Effects of induced rumen acidosis on the fecal shedding of Escherichia coli in lactating dairy cattle [Honors thesis]. Columbus, Ohio: The Ohio State University; 2012.
21. Pollitt CC. Equine laminitis: A revised pathophysiology. AAEP Proceedings 1999; 45:188-192.
22. Oetzel GR. Introduction to Ruminal Acidosis in Dairy Cattle Preconvention. Dairy Herd Problem Investigation Strategies. American Association of Bovine Practitioners 36th Annual Conference, Columbus 2003; 1-11.23. Kirker-Head CA, Stephens KA, Toal RL, Goble DO. Circulatory and blood gas changes accompanying the development and treatment of induced laminitis. J Eq Vet Science 1986; 6(6):293-301.
24. Verma AK. The interpretation of arterial blood gases. Aust Prescr 2010; 33(4):124-129.
25. Carreau A, El Hafny-Rahbi B, Matejuk A, Grillon C, Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J Cell Mol Med 2011; 15(6):1239-1253
26. Yang W, Hafez T, Thompson CS, Mikhailidis DP, Davidson BR, Winslet MC, Seifalian AM. Direct measurement of hepatic tissue hypoxia by using a novel tcpO2/pCO2 monitoring system in comparison with near-infrared spectroscopy. Liver Int 2003; 23(3):163-170.
27. Rodrigues M, Deschk M, Santos GGF, Perri SHV, Merenda VR, Hussni CA. et al. Avaliação das características do líquido ruminal, hemogasometria, atividade pedométrica e diagnóstico de laminite subclínica em vacas leiteiras. Pesq Vet Bras 2013; 33(1):99-106.
28. Akca O, Melischek M, Scheck T, Hellwagner K, Arkilic CF, Kurz A et al. Postoperative pain and subcutaneous oxygen tension. Lancet 1999; 354(3):41-42.