ORIGINAL
Interpretation of doxycycline+chloroquine dual therapy for A. phagocytophilum infection in dogs
Interpretación de terapia dual de doxicilina+cloroquina para la infección con A. phagocytophilum en perros
Kerem Ural, Ph.D.
1Adnan Menderes University, Faculty of Veterinary, Department of Internal Medicine, Isikli, Aydin, Turkey.
*Correspondence: uralkerem@gmail.com
Received: March 2014; Accepted: January 2015.
ABSTRACT
Objectives. A. phagocytophilum, an obligate intracellular pathogen, is a well-known agent causing granulocytic infections in both animals and humans. The purpose of the present study was to describe clinical course and consequences of Canine Granulocyctic Anaplasmosis among dogs in Aydin province, Turkey with special reference to hematological alterations and possible interpretations of doxycycline+chloroquine dual therapy. Materials and methods. A controlled clinical trial was carried out on 14 dogs referred and diagnosed as Canine Granulocyctic Anaplasmosis within Snap 4dx test. Relevant haematological data were recorded before (day 0) and after treatment (day 30) in both groups. Group I (n=7) were adminestered doxycycline (10 mg/kg q 12 h via oral route for 14 days) and chloroquine (2.5 mg/kg q 12 h for 14 days) and group II (n=7) received only doxycycline (10 mg/kg q 12 h for 14 days via oral route) therapy. Results. Doxycycline treatment hasten resolution of clinical signs in all dogs in about 2 to 7 days. There was no statistically significant differences among hematological variances detected (p>0.05). Conclusions. It may suggest that in conjunction with doxycycline chloroquine may have helped to speed up relevant clinical signs of CGA.
Key words: Anaplasmosis, Canine, chloroquine, doxycycline, granulocyctic (Source: CAB).
RESUMEN
Objetivo. A. phagocytophilum, un patógeno intracelular obligado, es un agente ampliamente conocido que causa infecciones granulocíticas tanto en animales como en humanos. El propósito del presente estudio fue describir la evolución clínica y las consecuencias de la Anaplasmosis Granulocítica Canina en perros de la provincia de Aydin, Turquía, con especial referencia a las alteraciones hematológicas y a las posibles interpretaciones de una terapia dual de doxicilina+cloroquina. Materiales y métodos. Se realizó un estudio clínico controlado en 14 perros remitidos y diagnosticados con Anaplasmosis Granulocítica Canina usando de una prueba Snap 4dx. Se registraron datos hematológicos pertinentes antes (día 0) y después del tratamiento (día 30) en ambos grupos. Al Grupo I (n=7) se le administró doxicilina (10 mg/kg q 12 h por vía oral durante 14 días) y cloroquina (2.5 mg/kg q 12 h durante 14 días), mientras que el Grupo II (n=7) recibió una terapia únicamente con doxicilina (10 mg/kg q 12 h por vía oral durante 14 días). Resultados. La doxicilina aceleró la resolución de los signos clínicos en todos los perros en un periodo de aproximadamente 2 a 7 días. No se detectaron diferencias estadísticas significativas entre las variaciones hematológicas (p>0.05). Conclusiones. Lo anterior puede sugerir que, conjuntamente con la doxicilina, la cloroquina puede haber ayudado a acelerar los signos clínicos pertinentes de la Anaplasmosis Granulocítica Canina (AGC).
Palabras clave: Anaplasmosis, canino, doxycycline, chloroquine, granulocyctico (Fuente: CAB).
INTRODUCTION
The canine vector-borne infectious diseases are emerging problems in veterinary medicine, besides the zoonotic potential of the latter causative agents may carry of importance for human health (1). The vector-borne canine diseases, especially human granulocytic ehrlichiosis caused by Anaplasma phagocytophilum (A. phagocytophilum) is frequently observed worldwide. Ticks are well recognized as the main method of transmission of the HE.
Anaplasma phagocytophilum is a well recognized agent of granulocytic anaplasmosis, possessing influence on neutrophils and rarely eosinophils (2). A. phagocytophilum infections can be detected either directly in blood smears (morulae in granulocytes) or by PCR or indirectly by serology. Many laboratories perform serological testing for IgG antibodies by using indirect immunofluorescent antibody techniques (IFAT).
Regarding human granulocytic anaplasmosis, diagnosis rely upon relevant clinical signs and laboratory analysis as follows; (i) microscobic morulae observation among neutrophils on satined blood smears along with antibody titer positivity against A. phagocytophilum; (ii) a 4-fold antibody titer elevation/reducing in 1 month; (iii) PCR positivity; or (iv) A. phagocytophilum identification and isolation in blood sample (3). Aforementioned criteria may also be adapted to dogs, apart from microbiological isolation (2,4-6).
Doxycycline is the armoured therapy option for Canine Granulocyctic Anaplasmosis (CGA). Indeed further research are warranted in an attempt to investigate any adjuvant or combined therapy alternatives for better releiving the hematopathogical and clinical signs. The aim of this study was to analyze clinical consequences of CGA among dogs, besides to scrutinize hematological alterations and interpretation of doxycycline+ chloroquine dual therapy.
MATERIAL AND METHODS
Conclusion criteria, sampling. The present study was enrolled among 14 dogs referred to the Small Animal Clinics at the Department of Internal Medicine, Faculty of Veterinary, Adnan Menderes University and privately owned small animal clinics in Aydin province between 2013 March-2014 February. All diseased dogs, referred with histories of at least one of the clinical signs involving fever, anorexia, weight loss, fever, generalized lymphadenopathy, arthropathy, muscle weakness, epistaxis and distal limb edema were evaluated using a canine point-of-care ELISA kit for diagnosis of naturally occuring vector borne diseases (SNAP 4Dx, IDEXX Laboratories, USA). Informed written consent was obtained from all of the dogs owners prior to enrolment of the dogs participated in study (n=14, at the age of 16 months to 4 years, 8 male, 6 female).
Laboratory analyses
Haematological analysis. Blood samples were withdrawn from vena cephalica antebrachii into anticoagulated (EDTA) tubes. Complete blood counts were performed on referral within Abacus Junior Vet hematology analyzer.
Serological analysis. Sera samples were tested within ELISA kit (SNAP 4Dx, IDEXX Laboratories, USA) in an attempt to diagnose antibodies against A. phagocytophilum, Ehrlichia canis, and Borrellia burgdorferi and antigen of Dirofilaria immitis, according to the protocol listed in the product insert. This assay detects antibodies reacting to immunodominant protein (msp2) of A. phagocytophilum. Only dogs naturally and mono-infected with A. phagocytophilum were enrolled in the present study. The test results were recorded in an Excel spreadsheet.
Treatment procedure. Fourteen dogs with a diagnosis of CGA were randomly enrolled into 2 groups. Treatment in both groups involved peroral administration. Group I (n=7) received doxycycline [(Monodoks capsule, 100 mg, Deva, Turkey) (10 mg/kg q 12 h for 14 days)] + chloroquine [(Kutlu tablet, 250 mg, Abdi İbrahim, Turkey) (2.5 mg/kg 12 h for 14 days)] and group II (n = 7) received mono-doxycycline (10 mg/kg q 12 h for 14 days) therapy.
Statistical analysis. Data were analyzed using the Mann-Whitney U (inter-group comparison) and the Wilcoxon test (intra-group comparison) procedures.
RESULTS
Serological results. Sera samples were tested within ELISA kit (SNAP 4Dx, IDEXX Laboratories, USA) revealed antibodies reacting to immunodominant protein (msp2) of A. phagocytophilum in all cases.
Clinical cure. Treatment in both groups resulted in rapid resolution of clinical signs in all dogs in about 2 to 7 days. Although not significant and statistically not important (data not shown) dogs enrolled in group I, that were dual treated, presented a more rapid clinical cure. Treatment in both groups resulted with clinical remission regarding aforementioned clinical signs. On day 30 no fever was evident in treated dogs. Spontaneous and gradual regression of enlarged lymp nodes occurred in 4 to 12 days in both groups. Finnaly on day 30 all dogs presented complete clinical recovery.
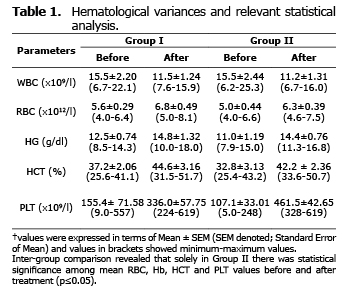
Analysis for relevant hematological variables. No significant difference was found among the mean hematological values of the two groups before and after treatment (p>0.05) (Table 1). Intra-group comparison revealed that there was no significant difference between mean values of hematological variances before and after treatment (p>0.05). Solely in doxycycline group showed significantly altered mean values regarding RBC, Hb, HCT and PLT before and after treatment (p≤0.05).
DISCUSSION
A. phagocytophilum, capable of infecting granulocytes, and so far neutrophils and eosinophils (7), frequently cause an acute febrile systemic illness, namely CGA. In an attempt to make precise diagnostic tests for CGA microscopic detection of specific morulae, anti-Anaplasma IgM and IgG antibody detection and PCR analysis, which is most reliable for early diagnosis (8).
Recent exposure to A. phagocytophilum may be determined in dogs and human by acute and convalescent serologic testing, to those of IFA assays (9). IgG class antibodies primarily are detectable following 8 days after first exposure, corresponding to 2–5 days after morulae presence. It is therefore may be suggested that antibody detection may not be possible during acute infection (7). Seronegative dogs could probably have been recently infected; indeed a seropositive case could have been exposed to the etiological agent preceeding months, in association with elimination of infection thorugh immunity (10). Polymerase chain reaction (PCR) assays can be used to detect organism-specific DNA sequences in the blood during the early stages (11). Therefore, the present authors may suggest that seropositive dogs obtained in the present study may have been infected weeks to months earlier, similar to previous description (9).
The major flaw in the present research was that serological or molecular testing results at the time of diagnosis was not available, other than Snap 4 Dx tests. Dogs enrolled in the present study were analyzed on referral with a qualitative ELISA assay able to determine A. phagocytophilum antibody. The aforementioned Snap 4 Dx test platform determines antibodies directed against a synthetic A. phagocytophilum peptide of a major surface protein, namely p44/MSP2, with sensitivity and specificity of 99 and 100%, respectively, in comparison to an immunoflourescent assay (1).
During acute infection with vector-borne organisms, involving Anaplasma species, clinical signs may be evident before the dog has a measureable antibody response (10). From another point of view clinical signs usually present 1 or 2 weeks following tick transmission of Anaplasmosis, indeed.
The vast majority of exposed dogs may not develop overt clinical disease. According to the acute nature of the latter infection, about 40% of clinically ill dogs possess antibodies directed against CGA at the time of referral (11, 12). Thus therapy applications might not solely based on antibody testing, even if the results are negative or positive (10).
In a recent prospective study in which the present author (K.U.) was also involved, doxycycline-chloroquine combination therapy was successfull against Canine Monocytic Ehrlichiosis (13). The results of the latter study suggested doxycycline+chloroquine combination as efficacous for releiving of clinical signs during Canine Monocytic Ehrlichiosis. In the doxycycline+chloroquine group all of the hematological parameters analyzed returned to physiological levels after this treatment (13). The latter authors (13) also claimed that treatment with doxycycline may probably alleviate the systemic effects of the tumor necrosis factor alpha, a cytokine that plays a role in the pathogenesis of acute canine ehrlichiosis, by reducing or eliminating parasitemia load (14). Furthermore chloroquine may have possessed anti-inflammatory effects (15, 16) and may have helped lowering the proinflammatory cytokines, or may be of beneficial for reducing infection of microbiological agents (17).
In another study 18 clinically ill dogs diagnosed with CGA by use of Snap 4 Dx analyte and PCR application, were treated with doxycycline (5-8 mg/kg q 12 h, for 14-30 days), which resulted in rapid resolution (the vast majority of the dogs treated showed recovery in 1-2 days) of clinical signs (10). A 7-year-old Labrador retriever with nonspecific clinical signs was diagnosed within CGA based on serological studies and PCR testing. The dog recovered after treatment with tetracycline (750 mg, PO, q8h for 14 d) (18). Another case from Czech Republic, an 11-months-old male Golden Retriever developed the acute onset of fever, lameness, inappetence, depression, ataxia and reluctance to move, was diagnosed within Anaplasma phagocytophilum infection. Oral doxycycline treatment for 14 days at adosage of 10 mg/kg q 12 h resulted within resolution of clinical symptoms in six days (19).
Chloroquine is a 4-aminoquinoline derivative possessing antimalarial activity for treatment and prevention (20,21). It has advantages due to its safety and effective usage, with a reasonable price of economic significance (20,21). Regarding its 60 days half-live, chemoprophylactic effect occurs during the elimination phase (21,22). Chloroquine might be safe therapy option for anti-inflammatory diseases. Although in the present study immunological parameters were not analyzed, chloroquine might have helped for a faster clinical remission in conjunction with doxycycline, as reported previously (13). However regarding hematological parameters evaluated, there was no statistical difference among groups treated solely with doxycycline or doxycycline + chloroquine. It should not be unwise to draw conclusion that larger dog population infected with CGA must be involved in studies evaluating chloroquine or similar antiprotozoer/antiinflammatory drug trials in an attempt to better understanding adjuvant therapy. Regardless of the interpretation, it must be kept in mind that Anaplasmosis may affect each dog differently, so alternative therapy protocols, as shown in this study, might aid clinicians for dissolving related clinical signs resulting in better animal welfare.
REFERENCES
1. Carrade D, Foley J, Sullivan M, Foley CW, Sykes JE. Spatial distribution of seroprevalence for Anaplasma phagocytophilum, Borrelia burgdorferi, Ehrlichia canis, and Dirofilaria immitis in dogs in Washington, Oregon, and California. Vet Clin Pathol 2011; 40(3):293-302.
2. Dumler JS, Barbet AF, Bekker CP. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int J Syst Evol Microbiol 2001;5:2145–2165.
3. Bakken JS, Dumler S. Human granulocytic anaplasmosis. Infect Dis Clin N Am 2008; 22:443–448.
4. Bakken JS, Dumler JS. Clinical diagnosis and treatment of human granulocytotropic anaplasmosis. Ann N Y Acad Sci 2006; 1078:236–247.
5. Bjöersdorff A. Ehrlichiosis and anaplasmosis, Part II: Anaplasma phagocytophilum comb. nov. infection. . Arthropod borne Infectious Diseases of the Dog and Cat. Manson Publishing, London; 2005.
6. Courtney JW, Kostelnik LM, Zeidner NS, Massung RF. Multiplex real time PCR for detection of Anaplasma phagocytophilum and Borrelia burgdorferi. J Clin Microbiol 2004; 42:3164–3168.
7. Carrade DD, Foley JE, Borjesson DL, Sykes JE. Canine granulocytic anaplasmosis: a review. J Vet Intern Med 2009; 23(6):1129-1141.
8. Bjoersdorff A. Canine granulocytic ehrlichiosis due to Anaplasma phagocytophila. Guide to Major Vector-borne Diseases of Pets, Merial, France. 2002.
9. McQuiston JH, McCall CL, Nicholson WL. Ehrlichiosis and related infections. J Am Vet Med Assoc 2003; 223(12):1750–1756.
10. Eberts MD, Vissotto de Paiva Diniz PP, Beall MJ, Stillman BA, Chandrashekar R, Breitschwerdt EB. Typical and atypical manifestations of Anaplasma phagocytophilum infection in dogs. J Am Anim Hosp Assoc 2011; 47(6):86-94.
11. Kohn B, Silaghi C, Galkea D, Arndt G, Pfister K. Infections with Anaplasma phagocytophilum in dogs in Germany. Res Vet Sci 2011; 91:71-76.
12. Granick JL, Armstrong PJ, Bender JB. Anaplasma phagocytophilum infection in dogs: 34 cases (2000-2007). J Am Vet Med Assoc 2009; 234(12):1559–65.
13. Aysul N, Ural K, Cetinkaya H, Kuşkucu M, Toros G, Eren H, Durum C. Doxycycline-Chloroquine Combination for the Treatment of Canine Monocytic Ehrlichiosis. Acta Sci Vet 2012; 40(2):1031.
14. Faria JL, Munhoz TD, João CF, Vargas-Hernández G, André MR, Pereira WA, Machado RZ, Tinucci Costa M. Ehrlichia canis (Jaboticabal strain) induces the expression of TNF-a in leukocytes and splenocytes of experimentally infected dogs. Braz J Vet Parasitol 2011; 20(1):71-74.
15. Park J, Kwon D, Choi C, Oh JW, Benveniste EN. Chloroquine induces activation of nuclear factor kappa B and subsequent expression of pro-inflammatory cytokines by human astroglial cells. J Neurochem 2003; 84(6):1266-1274.
16. Weber S, Levitz SM. Chloroquine antagonizes the proinflammatory cytokine response to oppurtunistic fungi by alkalizing the fungal phagolysosome. J Infect Dis 2001; 183(6):935-942.
17. Wozniacka A, Lesiak A, Narbutt J, McCauliff DP, Sysa-Jedrzejowska A. Chloroquine treatment influences proinflammatory cytokine levels in systemic lupus erythematosus patients. Lupus 2006; 15(5):268-275.
18. Lester SJ, Breitschwerdt EB, Hegarty BC. Anaplasma phagocytophilum infection (granulocytic anaplasmosis) in a dog from Vancouver Island. Can Vet J 2005; 46(9):825-827.
19. O Melter, I Stehlik, H Kinska, I Volfova, V Ticha, D Hulinska. Infection with Anaplasma phagocytophilum in a young dog: a case report Vet Med 2007; 52(5):207–212.
20. AlKadi HO. Antimalarial drug toxicity: a review. Chemotherapy 2007; 53(6):385-391.
21. Petersen I, Eastman R, Lanzer M. Drug-resistant malaria: molecular mechanisms and implications for public health. FEBS Lett 2011; 585(11):1551-1562.
22. Stepniewska K, White NJ. Pharmacokinetic determinants of the window of selection for antimalarial drug resistance. Antimicrob Agents Chemother 2008; 52:1589-1596.