ORIGINAL
Fribrinolytic activity and gas production by Pleurotus ostreatus-IE8 and Fomes fomentarius - EUM1 in bagasse cane
Actividad fibrolítica y producción de gas por Pleurotus ostreatus-IE8 y Fomes fomentarius-EUM1 en bagazo de caña
Paulino Sánchez-Santillán,1,5 Ph.D, Marcos Meneses-Mayo,2* Ph.D, Luis Miranda-Romero,3 Ph.D, Eduardo Santellano-Estrada,4 Ph.D, Baldomero Alarcón-Zúñiga,3 Ph.D.
1Colegio de Postgraduados, Campus Montecillo. Carretera México-Texcoco, Km 36.5, Montecillo, Estado de México, México, C.P. 56230.
2Universidad Anáhuac México-Norte, Facultad de Ciencias de la Salud (Nutrición), Av. Universidad Anáhuac No 46, Colonia Lomas Anáhuac Huixquilucan, Estado de México, México, C.P. 52786.
3Universidad Autónoma Chapingo, Departamento de Zootecnia. Carretera México-Texcoco, Km 38.5, Chapingo, Estado de México, México, C.P. 56230.
4Universidad Autónoma de Chihuahua, Facultad de Zootecnia. Periférico Francisco R. Aldama, km 1, Chihuahua, Chihuahua, C.P. 31543.
5Universidad Autónoma de Guerrero, Unidad Académica de Medicina Veterinaria y Zootecnia N°2, Carretera Acapulco Pinotepa Nacional Kilómetro 197, Cuajinicuilapa, Guerrero, México, C.P. 41940.
*Correspondence: marcos.meneses@anahuac.mx
Received: February 2015; Acepted: May 2015.
ABSTRACT
Objective. To characterize the fibrolytic enzymatic activity of Pleurotus ostreatus-IE8 and Fomes fomentarius-EUM1 in sugarcane bagasse (BCA); to evaluation of the kinetics of in vitro production of BCA treated by solid fermentation (FS), crude enzyme extract (ECE) of P. ostreatus-IE8 and Fibrozyme®. Materials and methods. In fungi measured radial growth rate ( Vcr ) and biomass production in two culture media (with or without nitrogen source); activity of xylanases, cellulases and FS on BCA at 0, 7 and 15 d. The chemical analysis and kinetic analysis of in vitro gas production in 4 treatments (ECE adding enzymes obtained from the direct addition FS or FS ), witness (Fibrozyme®) and a control without addition and analyzed by a was completely randomized design. Results. Xylanases (7 d ) showed 6.32 and 5.50 UI g-1 initial substrate dry weight (SSi) for fungi P. ostreatus-IE8 and F. fomentarius-EUM1 , respectively ; P. ostreatus-IE8 scored higher activity of laccases (10.65 g-1 UI SSi) and F. fomentarius-EUM1 (1.90 UI g-1 SSi) cellulases. The ECE of P. ostreatus-IE8 and commercial enzyme did not differences (p>0.05). In the chemical composition or the gas production kinetics. The 4 treatments evaluated decreased values of the variables measured in the kinetics of gas production compared to the control (p≤0.05). Conclusions. The ECE of P. ostreatus-IE8 was similar to commercial enzyme degradation in vitro, so it is feasible to use pre-digest high fiber products.
Key words: Fibrolytic enzymes, in vitro digestibility, solid fermentation, sugarcane bagasse (Source: DeCS).
Objetivos. Caracterizar la actividad enzimática fibrolítica de Pleurotus ostreatus-IE8 y Fomes fomentarius-EUM1 en bagazo de caña de azúcar (BCA) y evaluar la cinética de producción de gas in vitro del BCA por fermentación sólida (FS) o con extractos crudos enzimáticos (ECE) de P. ostreatus-IE8 o Fibrozyme®. Materiales y métodos. En los hongos de estudio se evaluó la velocidad de crecimiento radial (Vcr) y producción de biomasa en dos medios de cultivo (con o sin fuente de nitrógeno); actividad de xilanasas, celulasas y lacasas de la FS sobre BCA a 0, 7 y 15 d. El análisis químico y cinética de producción de gas in vitro en 4 tratamientos (proceso de FS o adición de enzimas obtenidas de ECE de la FS), un testigo (Fibrozyme®) y un control sin adición de enzimas, todo ello se analizó en un diseño completamente al azar. Resultados. Las xilanasas (7 d) mostraron 6.32 y 5.50 UI g-1 sustrato seco inicial (SSi) en P. ostreatus-IE8 y F. fomentarius-EUM1, respectivamente. P. ostreatus-IE8 mostró mayor actividad lacasa (10.65 UI g-1 SSi) y F. fomentarius-EUM1 (1.90 UI g-1 SSi) de celulasas. El ECE de P. ostreatus-IE8 y Fibrozyme® no presentaron diferencias (p>0.05) en la composición química ni en la cinética de producción de gas. Los 4 tratamientos evaluados disminuyeron los valores de las variables medidas en la cinética de producción de gas in vitro respecto al testigo (p≤0.05). Conclusiones. El ECE de P. ostreatus-IE8 fue similar a Fibrozyme® en la degradación in vitro, indicando su viabilidad y uso para pre-digerir subproductos altos en fibra.
Palabras clave: Bagazo de caña de azúcar, digestibilidad in vitro, enzimas fibrolíticas, fermentación sólida (Fuente: DeCS).
INTRODUCTION
The cell wall composition of sugarcane bagasse (SCB) has repercussions in ruminal digestion, considered to be relatively slow and incomplete. The nutritional value of SCB is low, and adding it as an ingredient to feed increases production costs. Biotechnological studies have shown that a combination of physical, chemical and biological treatments increase digestibility and nutritional quality of agro industrial byproducts (including SCB). Results are vary though (1). Okano et al (2) indicates that an option to increase the chemical availability of nutrients and the assimilation of byproducts of lignocellulosic fungi is solid fermentation (SF) and the use of fibrolytic exogenous enzymes, natural to these microorganisms and that are produced through liquid fermentation or in solid state (3).
The physiological and morphological characteristics of filamentous fungi allow for a good production of fibrolytic extracellular enzymes through SSF. Cellulase are enzymes that hydrolyze glyosidic bonds β-1,4 found in polysaccharides of cellulose. Xylanase acts in synergy and in sequence on the xylan structure to degrade it into constitutive sugar (4), and laccase, are polyphenol oxidase enzymes that oxidize phenolic compounds, the main element in lignin (5). The objective of this research is to characterize the fibrolytic enzymatic activity of a P. ostreatus-IE8 and F. fomentarius-EUM1culture in sugarcane bagasse; characterize the chemical composition and in vitro fermentation of SCB treated by SF, resulting in CEE of P. ostreatus-IE8 and to compare it with a commercial extract.
MATERIALS AND METHODS
Research Site. The investigation was conducted in the Microbiology Laboratory of the Zootechnic Department at the Universidad Autonoma Chapingo, and the Colegio de Posgraduados, both in the State of Mexico.
Inoculation. Two strains of Pleurotus ostreatus-IE8 and Fomes fomentarius-EUM1were used, both with the capacity to produce lignocellulosic enzymes. The EUM1 strain was taken from cultures available at the Universidad Autonoma Metropolitana and strain IE8 from the Colegio de Posgraduados.
Culture Medium. The strains were reactivated in an agar-malt extract and propagated in an initiating substrate using a mixture of sorghum grain and SCB in a ratio of 80:20, to be used later in the SF with SCB.
Experiments. Two experiments were conducted: The first one evaluated the enzymatic activity, radial speed growth (rsg), and biomass of the P. ostreatus-IE8 and F. fomentarius-EUM1 fungi strain. The second one determined the kinetics of in vitro fermentation using the gas production technique (6) of the SCB treated for 7 d with SF with the P. ostreatus-IE8 strain - evaluation of the CEE for 7 d with P. ostreatus-IE8-. These were compared with a commercial enzyme (Fibrozyme®, Alltech Inc., Nicholasville, KY) and a control, as well as the chemical composition of the treatments, controls and study control.
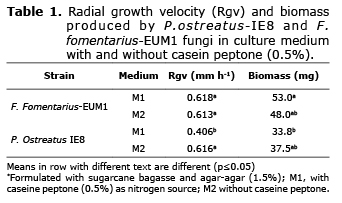
Determining the radial speed growth (rsg) and the dry matter of biomass. The rsg for both strains was measured for 7d in two culture mediums using the Trinci method (7): Medium 1 (M1) with sugarcane bagasse extract (BE) obtained by boiling 100 g of SCB in 1 L of distilled water for 30 min. Agar-agar (1.5%) and caseine peptone (0.5%) were added. Medium 2 (M2) had BE and agar-agar (1.5%) without caseine peptone. The P. ostreatus strain was incubated at 25°C and the F. fomentarius-EUM1 strain at 36°C. The dry mass of the biomass that grew after 7 days of incubation in mediums M1 and M2 were separated from the agar boiling it in distilled water and drying it for 24 hours at 60°C. The effect of mycelial growth on the culture methods evaluated (M1 and M2) was analyzed in a completely randomized experimental design with a 22 factorial arrangement. Data was analyzed with the GLM procedure of SAS® and means were compared with Tukey test (p≤0.05) to differentiate groups.
Solid medium fermentation. SF with each strain was done by placing 40 g of SCB (0.5 cm Ø and 85% moisture) in an Erlenmeyer flask. It was then sterilized for 15 min at 15 PSI and 121°C. The inoculation was done with 10 agar-malt extract cylinders with mycelium (1 cm Ø) of P. ostreatus-IE8 incubated at 25°C and 36°C for F. fomentarius-EUM1. In both cases the SF was evaluated at 0.7 and 15 d.
Determining enzymatic activity. In both strains the crude enzymatic extracts (CEE) obtained from SF at 0.7 and 15 days of fermentation were evaluated (four independent samples were gathered per day). The CEE was obtained by macerating the solid ferment of each strain for 20 min in distilled water at a ratio of 1:1.5. This was filtered and pressed by centrifugation for 20 min at 12.000 rpm at 4°C. Xylanase and cellulose activities were determined with the Miller method (9), laccase with ABTS according to Klis et al (10) and extracellular protein following the Bradford method (11). The experimental design used separate areas, divided in time, and was distributed randomly. Data was analyzed with the SAS® GLM procedure (8), and means was compared with the Tukey test (p≤0.05) to differentiate days of FS and experimental strains. The extracellular protein and laccase variables were transformed at logarithmic scale (y+1) to ensure data normality.
Enzymatic activity was expressed in international units (IU) per gram of initial dry substrate (IDS) in which one IU was defined as the quantity of enzymes released by 1 µmol xylose or glucose per min under the reaction conditions indicated.
Evaluation of in vitro kinetic fermentation of SCB. Four treatments were evaluated, one control, each in a Erlenmeyer flask with 100 g SCB as substrate (0.5 cm Ø and 85% moisture), sterilized for 20 min at 15 PSI and 121°C. A determined amount of freshly collected microbial inoculum, SCB fermented for 7 d, CEE from the 7th day SF, commercial cellulase (Fibrozyme®) as control and a control without enzymes. Treatments were classified as: FSi: 5 g of freshly collected inoculum from strain P. ostreatus-IE8; FSc: 5 g of SF (7d) in SCB with strain P.ostreatus-IE8; FS-ECE: 5 g of freshly collected inoculum from strain P. ostreatus-IE8 and 1.9 mL of CEE from SF (7d) from the same strain. CEE: 1.9 mL of CEE from FS (7d) of strain P. ostreatus-IE8; Control: 0.2 mL of commercial enzyme (Fibrozyme®); control: SCB without inoculum. The inoculum P. ostreatus-IE8 was obtained by spreading the strain on sorghum grain-SCB substrate in a proportion of 80:20, respectively. The SF and CEE were obtained at 7 d fermentation with P. ostreatus-IE8. The 7d Solid Fermented CEE and the commercial xylanase both showed the same results of 8.5 IU of xylanase; corresponding to diluting 1 g of commercial xylanase in 10 mL of distilled water (1 g kg-1 MS). The CEE treatments (1.9 Ml) had: 1.7 IU of xylanase and 0.01 cellulase. All samples were fermented for 7 d at 25°C, they were dried for 96 h at 60°C for nutrient analysis. An analysis was made on: dried matter (DM), ash, organic matter (OM), crude protein (CP), hemicellulose, cellulose, lignin acid detergent (LAD) according to AOAC (12), neutral detergent fiber (NDF) and acid detergent fiber (ADF) according to Van Soest, et al (13).
The kinetics of gas production was done with the Menke and Steigass method (6). A 0.5 g sample of a treatment and 90 mL of standardized ruminant inoculum were placed in ambar glasses with a flow of CO2. The glasses were placed on a double boiler at 39°C. Gas pressure was measured at 0, 2,4,10, 14, 18, 24, 30, 36, 48, 60 and 72 h of incubation. The transformation from pressure to gas volume was adjusted with a lineal regression model. The gas production scale (S), maximum volume of produced gas (Vm) and lag time (L) were obtained through the logistic model Va=Vm/ (1+ exp(2-4*S*(T-L))) (14). The final residues were filtered in ANKOM bags and dried for 24 h at 60°C. DM degradation (DEGMS) and real degradation (DEGV) (15) were calculated.
The experimental design was a random element. Data was analyzed using the GLM procedure of SAS® (8). Means were compared with the Tukey test (p≤0.05). Correlation was calculated using the CORR (8) procedure. Variables L, DEGMS and DEGV were made into square root, while %OM, %AFD and % hemicellulose were transformed using a logarithmic scale (y+1) to fulfill data normality requirements.
RESULTS
The RGR of P. ostreatus-IE8 in culture medium without peptone (M2) was superior (p≤0.05) to culture medium with peptone (M1), while fomentarius-EUM1did not show any difference between the culture mediums evaluated (p>0.05). Strains comparison did not show significant differences either (p>0.05). The biomass content was higher for F. fomentarius-EUM1 (p≤0.05) in both culture mediums (Table 1).
Table 2 shows that both fungi strains at 7d had increased enzyme activity, xylanase concentration did not vary (p>0.05) between strains on the 7d of SF. The P. ostreatus-IE8 strain had a greater laccase activity (10.65 UI g-1 SSi) at 7d, while F. fomentarius-EUM1 had greater cellulase activity (1.90 UI g-1 SSi) at 7d of SF. The P. ostreatus-IE8 strain produced a higher concentration of extracellular protein coinciding with the 7d of SF (0.52 mg g-1 SSi; Table 2).
Table 3 shows the results of the evolution of maximum gas production volume (Vm), dried matter degradation (DEGMS), and real degradation (DEGV). FSi, FSc and SF-CEE methods had the lowest Vm, DEGM and DEGV values, with no difference among them (p>0.05). CEE treatments and control methods produced values higher than 100 mL g MS-1, with control showing the highest Vm (123.80) mL g MS-1). The CEE and control method did not increase DEGMS and DEGV when compared to the control method.
The fractions of NDF, ADF and ash from all methods decreased when compared to the control method (p≤0.05). In contrast, the fractions of LAD, CP and OM increased in comparison to the control method. The methods with added SF of 7d had higher values of LAD, CP and ash (p≤0.05) in relation to those with added enzymes (CEE and control) (Table 4).
DISCUSION
Basidiomycete fungi P. ostreatus and F. fomentarius have the capacity to simultaneously produce hydrolytic and lignolitic enzymes by SF (16). Marquez et al (17) conducted an FS test for 14 d at an incubation temperature of 34°C with F. fomentarius-EUM1 on SCB and 80% moisture. Xilanase (147.27 UI g-1 SSi) and cellulase (8.51 UI g-1 SSi) activity was reported, but lower on laccase (3.45 UI g-1 SSi). Membrillo et al (18) used P. ostreatus-IE8 in a SF for 8 d on SCB with a particle size of 2.9 mm and 80% moisture. The xylanase activity was reduced (5.79 U g-1 SSi) as well as laccase (0.021 U g-1 SSi).
The SF process has important considerations: particle size, type of substrate, microorganisms, nature of inoculum, temperature and duration (3,4). For those reasons, and based on the results of this research, it is important to consider the enzyme-substrate affinity for this type of biotechnological developments because of the great variations that exist. The chemical characteristics of SCB make it possible for the P. ostreatus-IE8 strain to have great potential to produce laccase enzymes and act specifically on the lignin, while F. fometarius has a greater cellulase production capacity, acting directly on the cellulose on the cell wall of fibrous forages used to feed ruminants (1)
It is unknown if adding complex organic nitrogen sources like casein peptone can have an inhibiting effect on radial growth speed and biomass production, given that existing literature only includes mineral nitrogen. Membrillo et al (18) used urea and ammonia sulfate as a source of organic nitrogen in a malt extract medium to grow P. ostreatus-IE8, resulting in inferior rsg (0.234 y 0.308 mm h-1). Levin et al (19) tried using different nitrogen sources for the production of laccase by three basidiomycetes fungi, without finding any differences in the laccase production patterns given the medium composition. Therefore, the addition of nitrogenized sources to culture mediums for the growth of basidiomycete fungi does not increase the fungal rsg or enzyme production (18, 19).
The kinetics of in vitro gas production results from the anaerobic fermentation of soluble carbohydrates or cellular wall with microorganisms from the rumen. The FSi, FS-CEE treatments presented less values in the production kinetics of in vitro gas (Table 3) because they result from a fermentation process where P. ostreatus-IE8 consumes soluble carbohydrates and hemicellulose as primary sources of energy for growth (2,20). CEE and the control treatment had a larger amount of carbohydrates available because they were not predigested. The CEE and control treatment showed different behaviors in the gas production kinetics when compared with the control treatment, even when the enzymes produced by SF promotes greater availability of structural and soluble carbohydrates on the cell wall as a result of the degradation of the substrate, producing more gas in in vitro studies (20).
The increase of LAD and ash fraction, as well as the decrease of the NDF, ADF, cellulose and OM fraction in the FSi, FSc and SF-CEE treatments are attributed to the fact that the P. ostreatus-IE8 fungi consumes a large amount of carbohydrates, (2) necessary for its development. The increase of CP in the FSi, FSc and SF-CEE treatments is the result of the increase of microbial protein produced directly in the development of the P. ostreatus-IE8 fungi on SCB in the SF process (21). The relation between chemical composition and variables in the kinetics of in vitro gas production are used to estimate the digestibility of a substrate by a ruminant (22). The Vm of gas was positively correlated with NDF, hemicellulose and cellulose, but negatively with LAD (Table 5). Andres et al (23) reported similar findings. His correlations indicate the same tendencies as this study. The negative correlation of CP with the kinetic variable of gas production is explained because the protein is used in the biosynthesis of microbial protein, and in case microorganisms increase their immediate demand for energy, it catabolizes into an energy source. The positive correlation between DEGMS and DEGV with NDF, cellulose and hemicellulose, but negative with LAD and CP are due to the double fermentation process (solid and anaerobic) that the SCB underwent. Bindelle et al (22) reported negative correlations with NDF and positive with CP because they did not apply to the substrates any sort of fermentation previous to the in vitro gas production, allowing for a larger amount of carbohydrates.
This research shows that the mycelial growth of the Pleurotus ostreatus-IE8 and Fomes fomentarius-EUM1fungi increases when SCB is added to a conventional culture medium. F. fomentarius-EUM1 and P. ostreatus-IE8 strains have higher enzymatic activity at 7 d of fermentation on SCB. The crude enzymatic extract (7 d) of P. ostreatus-IE8 on SCB showed similar effects on the kinetics of in vitro gas production (72 h incubation) as the commercial enzymatic product (Fibrozyme®). The application of lignocellulolytic or a solid fermentation on SCB reduces the production of in vitro gas and the real degradation of substrate.
REFERENCES
1. Sánchez-Santillán P, Meneses MM, Torres-Salado N. Production of Lignocellulolytic Enzymes with Pleurotus ostreatus-IE8 by Solid Fermentation and Its Effect on the Chemical Composition of Sugarcane Bagasse. Life Sci J 2015; 12(2s):37-41.
2. Okano K, Fukui S, Kitao R, Usagawa T. Effects of culture length of Pleurotus eryngii grown on sugarcane bagasse on in vitro digestibility and chemical composition. Anim Feed Sci Technol 2007; 136(3-4):240-247.
3. Cuervo L, Folch JL, Quiroz RE. Lignocelulosa como Fuente de azúcares para la producción de etanol. BioTecnología 2009; 13(3):11-25.
4. Kumar SS, Sczakas G, Soccol CR, and Pandey A. Production of Enzymes by Solid-State Fermentation in Current Developments in Solid-State Fermentation. 1a ed. New York: Springer; 2008.
5. Loera CO, Pérez PMCI, Barbosa RJR, and Villaseñor OF. Laccases. In: Guevara-González RG, Torres-Pacheco I, editors. Advances in Agricultural and Food Biotechnololy. Kerela, Indía: Cabi; 2006.
6. Menke KH and Steingas H. Estimation of the energetic feed value obtained from Chemical analysis and in vitro gas production using rumen fluid. Anim Res Develop 1988; 28(1):7-55.
7. Trinci APJ. A kinetic study of the growth of Aspergillus nidulans and other fungi. J Gen Microbiol 1969; 57(1):11-24.
8. SAS. SAS/STAT Sofware. Version 9.3. Cary, NC SAS, USA: Institute Inc; 2011.
9. Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Biochem 1959; 31(3):426-428.
10. Klis M, Rogalski J, Bilewicz R. Voltammetric determination of catalytic reaction parameters of laccase bases on electrooxidation of hydroquinone and ABTS. Bioelectrochemistry 2007; 71(1):2-7.
11. Bradford MM. A rapid and sensitive for the quantitation of microgram quantities of protein-dye binding. Anal Biochem 1976; 72(1-2):248-254.
12. A.O.A.C. Official Methods of Analysis (18th Ed). Washington D.C.: A.O.A.C International; 2005.
13. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 1991; 74(10):3583-3597.
14. Schofiel P, and Pell AN. Measurement and kinetic analysis of the neutral detergent-soluble carbohydrate fraction of legumes y grasses. J Anim Sci 1995; 73(11): 3455-3463.
15. Blümmel M, Cone JW, Van Gelber AH, Nshalai I, Umunna NN, Makkar HPS, et al. Prediction of forage intake using in vitro gas production methods: Comparison of multiphase fermentation kinetics measured in an automated gas test, and combined gas volume and substrate degradability measurements in a manual syringe system. Anim Feed Sci Technol 2005; 123-124(1):517-526.
16. Elisashvili V, Kachlishvili E and Penninck M. Effect of growth substrate, method of fermentation, and nitrogen source on lignocelluloses-degrading enzymes production by white-rot basidiomycetes. J Ind Microbiol Biotechnol 2008; 35(11):1531-1538.
17. Márquez AAT, Mendoza MGD, González MSS, Buntins DSE y Loera CO. Actividad fibrolítica de enzimas producidas por Trametes sp EUM1, Pleurotus ostreatus IE8 y Aspergillus niger AD96.4 en fermentación sólida. Interciencia 2007; 32(11):780-785.
18. Membrillo I, Sánchez C, Meneses M, Favela E, Loera O. Effect of substrate particle size and additional nitrogen source on production of lignocellulolytic enzymes by Pleurotus ostreatus strains. Bioresource Technol 2008; 99(16):7842-7847.
19. Levin L, Melignani E, Ramos AM. Effect of nitrogen sources and vitamins on ligninolytic enzyme production by some white-rot fungi. Dye decolorization by selected culture filtrates. Bioresource Technol 2010; 101(12):4554-4563.
20. Peláez-Acero A, Meneses-Mayo M, Miranda-Romero LA, Ayala-Martínez M, Crosby-Galván MM, Loera-Corral O, y Megías-Rivas MD. Enzimas fibrolíticas producidas por fermentación en estado sólido para mejorar los ensilajes de caña de azúcar. Agrociencia 2011; 45(6):675-685.
21. Akinfemi A, Ogunwole OA, Ladipo MK, Adu OA, Osineye OM, and Apata ES. Enhacement of the nutritive value of maize leaf treated with white-rot fungi: Pleurotus sajur caju and Pleurotus pulmonarius, and the effects on chemical composition and in vitro digestibility. Prod Agric Technol 2008; 4(1):106-114.
22. Bindelle J, Ilunga Y, Delacollette M, Muland-Kayij M, Umba M, Kindele E and Buldgen A. Voluntary intake, chemical composition and in vitro digestibility of fresh forages fed to Guinea pigs in periurban rearing systems of Kinshasa (Democratic Republic of Congo). Trop Anim Health Prod 2007; 39(6):419-426.
23. Andrés S, Calleja A, López S, González JS, Rodríguez PL, Giráldez FJ. Prediction of gas production kinetic parameters of forages by chemical composition and near infrared reflectance spectroscopy. Anim Feed Sci Technol 2005; 123-124(1):487-499.