ORIGINAL
Hematological and clinical chemistry changes induced by acute stress during handling and capture of catfish (Ictalurus punctatus)
Cambios hematolágicos y bioquímicos provocados por estrés agudo causado por manejo y captura de bagre (Ictalurus punctatus)
Gabriel Aguirre-Guzman,1 Ph.D, Veránica Carvajal-de-la-Fuente,1* M.Sc, Miriam Neri-Coronado,1 MVZ, Jorge Loredo-Osti,1 MC, Jaime Luis Rábago-Castro,1 Ph.D.
1Universidad Autánoma de Tamaulipas, Facultad de Medicina Veterinaria y Zootecnia. Km 5 Carr. Victoria - Mante, Cd. Victoria, Tamaulipas, México.
*Correspondence: vcarvajal@uat.edu.mx
Received: August 2015; Accepted: January 2016
ABSTRACT
Objective. Evaluar los efectos del estrés agudo debido al manejo y captura sobre los parámetros hematolágicos y bioquímicos en bagre de canal (Ictalurus punctatus) bajo cultivo. Materiales y métodos. Los peces (200 g promedio) fueron mantenidos en tanques de cultivo y divididos en dos tratamientos, por duplicado, (n= 15 x 2 x 2 = 60 peces). Treinta bagres fueron expuestos por 5 min a estrés agudo (TE) por manejo y captura, mientras que otro grupo no (grupo control, TnE). Diez peces de cada tratamiento fueron colectados a las 0, 6, y 24 h post-estrés para la extraccián de sangre, los bagres del TnE fueron anestesiados durante su manejo y captura. Se evaluá el hemograma (método manual) y bioquímica sanguínea (espectrofotometría). Los resultados fueron analizados mediante la prueba de t student. Resultados. El contenido de eritrocitos, hematocrito, hemoglobina y glucosa de los animales TE fue significativamente mayor (p<0.05) a las 6 h post-estrés en comparacián de TnE. Las células inmune en peces TE disminuyeron a las 6 y 24 h post-estrés, siendo leucocitos y linfocitos significativamente menores en el TnE (p<0.05) a las 24 h post-estrés. Otros parámetros evaluados no presentaron diferencias significativas en lo largo del estudio. Conclusiones. Los resultados sugieren que varios indicadores hematolágicos y bioquímica sanguínea en los peces son alterados por el estrés agudo ocasionado por manejo y captura.
Key words: Bagre, bioquímica sanguínea, estrés, valores hematolágicos (Fuente:CAB).
Objetivos. Evaluation of hematological and biochemical parameters of culture channel catfish (Ictalurus punctatus) under acute stress by management and capture practice. Materials and methods. Fish (200 g mean) were maintained in culture tanks and divided in two treatments, in duplicate, (n=15x2x2=60 fishes). Thirty catfish were exposed for 5 min to acute stress (TE) by management and capture practice, while other group not (control group, TnE). 10 fish for treatment were collected at 0, 6, and 24 h post-stress for blood collection, where TnE fishes were anesthetized along work. Complete blood count (manual method) and blood biochemical (spectrophotometry) of fish samples were evaluated and their results were analyzed using a Student’s t-distribution. Results. The erythrocytes, hematocrit, hemoglobin and glucose level of TE animals was significantly higher (p<0.05) at 6 h post-stress, in comparison of TnE. Immune cells in fish TE decreased at 6 and 24 h post-stress, where leukocytes and lymphocytes were significantly lower that TnE (p < 0.05) at 24 h post-stress. Other evaluated parameters did not show significant differences along this study. Conclusions. Those results suggest that several hematological and blood biochemical parameters in fish changed by acute stress generated by management and capture practice.
Palabras clave: Blood parameters, blood biochemical, catfish, stress (Source:CAB).
INTRODUCTION
Aquaculture production has become very important in recent times to ensure food security, which has benefitted from the increasing number of species cultivated. Aquaculture, a constantly growing industry, provides about 50% of the aquatic foods consumed worldwide (1). Additionally, consumption of aquaculture products has increased from 10 kg to 19 kg per capita between 1960 and 2012 (1), representing about 17% of animal protein intake and constituting one of the most important sources of essential vitamins, nutrients and omega-3 acids (1).
Cultured channel catfish (Ictalurus punctatus) is a relevant species on a global scale, generally accepted in national and international markets due to its quality and nutritional value as well as its presentation (2). Over the last decade the production of cultured channel catfish (I. punctatus) has varied around the world (250.000 - 400.000 tons) and in Mexico (760−1.496 tons). Exploitation, ranging from extensive to intensive, of this as well as other sweet water fish depends on a variety of factors to reach maximum productive levels. However, achieving adequate production levels involves the presence and increase of a variety of stress elements (high densities with a subsequent deterioration in water quality, handling and capture practices, etc.) that could modify the physiological response of fish (3,4).
Studies of aquaculture species show that different levels of stress can lead to a decrease in growth, health, survival, and reproduction (5,6). Furthermore, it has been reported that stress can generate hormone abnormalities, alterations in blood glucose and changes in blood cell composition (7). However, the effect of stress on fish varies from one species to another, and much depends on the duration and magnitude of the stressor (8,9).
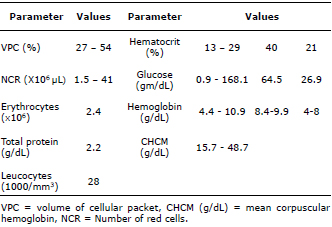
Information pertaining to blood and hematologic values in channel catfish is limited (Table 1) (10-13) and demonstrates that variations in these parameters exist, even though the stress factor was not considered in these studies. However, it is observed that increased stress results in immunosuppression which may favor disease, mortality and decreased production (14,15). Understanding how stress can affect fish physiology is important and is a way to support this industry’s development. The purpose of this study was to evaluate the effect of acute stress generated by handling and capture on hematology and chemical blood parameters in channel catfish (I. punctatus).
Table 1. Blood and chemistry values in channel catfish (Ictalurus punctatus) (10-13).
MATERIALS AND METHODS
Experimental animals and culture area. Sixty young channel catfish (200 g mean) from the commercial farm ACUMEX S.A. of C.V., Abasolo, Tamaulipas, Mexico were used. The fish were divided into two treatment groups (stressed or TE and not stressed or TnE) with 30 fish in each group. Each treatment was distributed in two cylindrical fiberglass tanks (192.4L) with fifteen fish in each tank. The fish were acclimated at 25°C for three weeks in culture tanks in sweet water from a deep well at the Dr. Norberto Treviño Zapata Faculty of Veterinary and Zootechnical Medicine (FMVZ). Two aeration stones were placed in each tank (Aquatic eco system, Model 2pS, USA) that were connected to an air blower (Aquatic eco-system, Model HP 4S, USA) to achieve a dissolved oxygen level of 5 ppm. Additionally, the water’s level of non-ionized ammonium (NH3) was below 0.01 mg/L. The fish were fed ad libitum without waste twice daily (0900 and 1700 hr.) with a commercial pellet diet (Agribrands Purina México S. A. C. V., Nutripec 3206 AP).
Applying stress to the fish. After the acclimation period, all fish were subjected to fasting for a period of 12 hours before the stress test. The TE catfish were exposed to acute stress through excessive intentional handling that consisted of pursuing and capturing the fish with a net and placing them in 20 L buckets for 5 min (16). The buckets were aerated and contained the same well water (25°C) that was in the culture tanks. At the end of the stress period, the fish were returned to their original tanks. To avoid stress and the resulting effects on their physiological base levels, the control group was not subjected to any kind of handling.
Sampling. To take the blood tests, ten fish from each treatment group (TE and TnE) were captured at 0, 6, 24 h post-stress. NETs (control) fish were anesthetized in culture tanks using 40 mg/L benzocaine for 3 to 5 min before being captured for sampling (17). Once anaesthetized and captured they were placed in a bucket with fresh well water (25°C) with continuous aeration and the same concentration of anesthetic. TE fish were caught in their culture tanks without anesthesia and treated the same way as the TnE group.
A blood sample (3 mL) of each catfish was collected by heart puncture in a Vacutainer® tube with lithium heparin. Samples were stored on ice and transported to the laboratory for clinical analysis. Each sample was divided into two parts; one of which was used for hematology, and the rest was centrifuged at 3000 rpm for 15 min at room temperature to extract plasma. The product was refrigerated (4°C) to later define blood chemistry (18).
Hemogram. This analysis was performed within 4 h post-sampling to avoid any disruption in blood cells (19). The hematocrit (Ht) was determined using a digital microcentrifuge (KHT-410E Kendal Import S.A.C Gemmy Taiwan); hemoglobin (Hb) using cyanmethemoglobin method and read in a spectrophotometer (Spectronic 20 Genesys, Spectronic Instruments, Rochester, NY, USA) at 546 nm (20). Erythrocyte indices: mean corpuscular volume (VCM) and mean corpuscular hemoglobin concentration (CHCM) were estimated using the formulas described by Noga (20). Total erythrocyte and leukocyte count was manually calculated using a Neubauer chamber (Superior 06-100-10 Optic Labor, Wertheim, Germany) using Natt-Herrick as diluent reagent at a dilution of 1:200 (20). The differential count was performed using blood that was labeled and stained with Wright dye. The slides were observed with an optical microscope (Carl Zeiss, Axiostar Plus, Germany) with an immersion objective (100x) following the technique suggested by Fijan (19).
Blood chemistry. To determine the concentrations of albumin, ALT (Alanine aminotransferase), creatinine, glucose, and FA (Alkaline Phosphatase) in plasma, a dry chemistry analyzer (Vet Test 8008, IDEXX Laboratories Inc., Maine, USA) was used (21). The concentration of total plasma proteins was obtained using a refractometer (American Optical TSM, Buffalo, NY, USA) at room temperature (22).
Statistical analysis. The results of the hematological parameters were represented by mean and standard deviations, and were compared between treatments (TE vs TNE) and time frames (0, 6, and 24 h). The statistical evaluation of these variables was performed using Student’s t-test for independent samples with a significance level of 0.05, and SAS statistical software v. 9.0 was used.
RESULTS
Table 2 shows the blood count results of channel catfish (I. punctatus) under stress (TE) and the no-stress or control group (TNE). It can be noted that the hematological parameters at time 0 post-stress showed no statistically significant differences (p>0.05) between TE and TNE (Table 2), except for the value of monocytes and eosinophils, where group TE shows a significantly higher value (p<0.05). All the immune system cells evaluated have higher assessed values in the TE group than in the TnE group. Only monocytes and eosinophils have significantly higher values (p<0.05), as noted above.
Table 2. Hematology parameters (mean±DE) of treated catfish (Ictalurus punctatus) (n=10).
In this study, parameters evaluated at 6 h post-stress for TE were significantly higher when compared with the parameters of TnE (p<0.05). Only erythrocytes, HCM and eosinophils showed higher values in TE compared with TnE, although they were insignificant (Table 2). Later, at 24 h post-stress, very similar results were observed between TE and TnE groups, showing no significant differences between them (p>0.05).
Comparing hematologic parameters over time (0, 6, and 24 h post-stress), it can be noted that the TnE group shows no significant differences (p>0.05) in any of its parameters (Table 2). The same applies to TE in regards to erythrocytes, hemoglobin, hematocrit, VCM and CHCM. However, immune system cells in TE show a tendency to decrease over time and have lower values at 24 h than at 0 h. Only leukocytes and lymphocytes revealed significantly lower values (p<0.05) at 24 h compared with 0 and 6 h post-stress (Table 2). Additionally, a clear difference between immune cells values in TnE and TE at different sampling times is observed.
Table 3 shows blood biochemistry values in channel catfish (I. punctatus) after being stressed. The detected values show that only glucose has a significantly higher value (p<0.05) at 6 h post-stress in TE compared to TnE. This difference is lost at 24 h. However, it can be seen that average glucose is always lower in TnE than TE (Table 3). All other values evaluated in the blood chemistry showed no significant differences between groups. Similarly, no significant differences (p> 0.05) were detected when comparing these parameters with regard to time (0, 6 and 24 h post-stress).
Table 3. Blood biochemistry (mean±DE) of treated catfish (Ictalurus punctatus) (n=10).
DISCUSSION
The results of the non-stressed group (TNE) are consistent with those previously reported (Table 1). It is interesting to note the lack of information that exists on this subject in channel catfish (I. punctatus), since these parameters are crucial to help predict the state of health during production. The values observed in stressed catfish (TE) are consistent with those reported in trout (Oncorhynchus mykiss) under acute stress where the blood count (erythrocytes, hemoglobin, hematocrit and VCM) was significantly higher (p<0.05) in TE than TnE (23-26). Pottinger et al (27) suggest that hematocrit could increase due to the action of catecholamines through smooth muscle contraction and the elastic fibers of the spleen capsule. This could generate a simultaneous increase in cell number and hemoglobin concentration, improving the O2 transport efficiency required to metabolize tissues exposed to acute stress (7,27).
The significant increase (p<0.05) of VCM observed at 6 h could be an early adaptation response to stress, with subsequent hemodilution caused by electrolyte loss and reduced osmolarity. However, when comparing this parameter with respect to time (0, 6 and 24 h post-stress), no significant differences (p>0.05) in this parameter were observed between TE and TnE. This suggests that increased hematocrit could be caused by an increase in the number of erythrocytes as a necessary response to carry more oxygen to the tissues when under conditions of acute stress, as indicated by Gámez-Manrique et al (28), who reported similar results.
Leukocytes, lymphocytes, heterophiles and monocytes significantly increased (p>0.05) at 6 h post-stress. Tort (29) points out that some immune system cells may increase as a precautionary measure or response to stressful stimuli. The results show that after the initial stimulus, TE fish show a decrease in the number of immune cells that can lead to further immunosuppression, as suggested (3, 4, 15, 29). How this immune system response works is still poorly understood; however, it suggests that it may be related to the release of cells from spleen, thymus and other tissues (29).
Comparing the average values of leukocytes and lymphocytes in the TE group at different time periods (0, 6, and 24 h post-stress) shows significantly lower values (p<0.05) at 24 h post−stress (Table 2). This is consistent with what Adeyemo et al (4) reported, who observed that African catfish (C. gariepinus) have lymphocytosis, eosinophilia and monocytosis when they are experimentally exposed to acute handling stress. The control group (TnE) showed no significant differences in any immune system cells at different post-stress times. It has been reported that during periods of stress the combination of cortisol and catecholamine produces an immunosuppressive effect on lymphocytes and monocytes (8). Furthermore, it has been reported that catecholamine increases heart rate and blood pressure, releasing a greater amount of white blood cells into circulation during the first hours (8, 30), as observed in this work.
Blood glucose, like cortisol, is a marker of the stress response metabolic rate (14), which has the advantage of being easy to evaluate with a narrower raising point (2 times) than cortisol (100 times) (7, 16, 24, 31). In this study, TnE glucose levels had a lower average than those observed in TE in the first two evaluations. This indicator is significantly higher (p<0.05) at 6 h post-stress, and holds steady at 24 h post-stress in both groups (Table 2). These detected values are consistent with those reported in channel catfish (I. punctatus) in Table 1 and also in trout (O. mykiss, 25) and tilapia (Oreochromis niloticus, 26). However, Davis et al (14) also report an increase in blood glucose at 6 and 0 h post-stress compared to the control group and the stressed group, respectively. Hyperglycemia observed in TE could be the result of glycogenolysis and glucogenogenesis, since both catecholamines and cortisol are involved in this effect. Since this increase was only observed at 6 h post-stress, it is suggested that the change was due to the effect of catecholamines since it has been proposed that glucose production is mediated in the short term by these hormones and in the long-term by cortisol (27).
The observed results suggest that acute stress alters blood parameters, immune cells, and blood chemistry in fish. These alterations may decrease significantly as time passes and are an important component to consider during production to prevent possible problems that negatively impact the health of cultivated fish.
Acknowledgements
To the Universidad Autánoma de Tamaulipas for their financial support (Proyecto UAT10-AGRO-0208).
REFERENCES
1. Farmer T, Grainger R, Plummer J, editors. The state of world fisheries and aquaculture. Opportunities and challenges. Rome, FAO; 2014.
2. Sanchez-Martinez JG, Aguirre-Guzman G, Cruz-Hernandez NI, Martinez-Burnes J, Perez-Castaneda R, Rabago-Castro JL, Vazquez-Sauceda ML. First detection of channel catfish virus associated with mortality of cultured catfish (Ictalurus punctatus, Rafinesque) in Mexico. Aquacult Res 2007; 38(13):1428-1431.
3. Gbore FA, Oginni O, Adewole AM, Aladenton JO. The effect of transportation and handling stress on haematology and plasma biochemistry in fingerlings of Clarias gariepinus and Tilapia zilli. World J Agr Sci 2006; 2(2):208-212.
4. Adeyemo O, Naigaga I, Alli R. Effect of handling and transportation on heamatology of African catfish (Clarias gariepinus). J Fish Sci 2009; 3(4):333-341.
5. Davis MW. Fish strass and mortality can be predicted using relax impairment. Fish Fish 2010; 11:1-11.
6. Eslamloo K, Falahatkar B. Variations of some physiological and immunological parameters in siberian sturgeon (Acipenser baerii, Brandt, 1869) subjected to an acute stressor. J Appl Anim Welf Sci 2014; 17(1):29-42.
7. Pottinger TG. The stress response in fish mechanisms, effects and measurement. Branson EJ, editor. Fish welfare. London: Blackwell Publishing; 2008.
8. Prunet P, Sturm A, Milla S. Multiple corticosteroid receptors in Wsh: from old ideas to new concepts. Gen Comp Endocrinol 2006; 147(1):17−23.
9. Schreck CB. Stress and fish reproduction: the roles of allostasis and hormesis. Gen Comp Endocrinol 2010; 165(3):549-556.
10. Iwama GK, Pickering AD, Sumpter JP, Schreck CB. editors. Effect of rearing condition on the health and physiological quality on fish in intensive culture. Fish stress and health in aquaculture. New York: Cambridge University Press; 2011.
11. Tavares-Dias M, Moraes FR. Haematological and biochemical reference intervals for farmed channel catfish. J Fish Biol 2007; 71(2):383−388.
12. Buentello JA, Reyes-Becerril M, Romero-Geraldo MJ, Ascencio-Valle FJ. Effects of dietary arginine on hematological parameters and innate immune function of channel catfish. J Aquat AniM Health 2007; 19(3):195-203
13. Taveras-Dias M, Moraes FR. Hematological and biochemical reference intervals for farmed channel catfish. J Fish Biol 2007; 71(2):383-388.
14. Davis KB. Management of physiological stress in finfish aquaculture. N Am J Aquacult 2006; 68(2):116-121.
15. Bilodeau AL, Small BC, Wise DJ, Wolters WR. Pathogen levels, lysozyme, and cortisol response in channel catfish with susceptibility differences to Edwardsiella ictaluri. Gen Comp Endocrinol 2005; 142(1-2):256-62.
16. Magnadáttir B. Innate immunity of fish (overview). Fish Shellfish Immun 2006; 20(2):137-151.
17. Kiessling A, Johansson D, Zahl IH, Samuelsen OB. Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration. Aquaculture. 2009; 286(3-4):301-308.
18. Minder EI, Schibli A, Mahrer D, Nesic P, Plüer K. Effects of different centrifugation conditions on clinical chemistry and immunology test results. BMC Clin Pathol 2011; 11(6):1-15.
19. Fijan N. Composition of main haematopoietic compartments in normal and bled channel catfish. J Fish Biol 2002; 60(5):1142−1154.
20. Noga EJ. Fish diseases and diagnosis and treatment. Wiley-Blackwell FAO. Iowa, USA. 2010.
21. Velisek J, Svobodova Z, Piackova V, Groch L, Nepejchalova L. Effects of clove oil anaesthesia on common carp (Cyprinus carpio L.). Vet Med, 2005; 50(6):269−275.
22. Riche M. Analysis of refractometry for determining total plasma protein in hybrid striped bass (Morone chrysops×M. saxatilis) at various salinities. Aquaculture 2007; 264(1-4):279-284.
23. Small BC, Bilodeau AL. Effects of cortisol and stress on channel catfish (Ictalurus punctatus) pathogen susceptibility and lysozyme activity following exposure to Edwardsiella ictaluri. Gen Comp Endocrinol 2008; 70(2):223-235
24. Iwama GK. The welfare of fish. Dis Aquat Organ 2007; 75(4):155-158.
25. Merkin GV, Roth B, Gjerstad C, Dahl-Paulsen E, Nortvedt R. Effect of pre-slaughter procedures on stress responses and some quality parameters. Aquaculture 2010; 304(1-4):231-235.
26. Welker TL, Lim C, Yildirim-Aksoy M, Klesius PH. Growth, immune function, and disease and stress resistance of juvenile Nile tilapia (Oreochromis niloticus) fed graded levels of bovine lactoferrin. Aquaculture 2007; 262(1):156−162.
27. Pottinger TG, Henrys PA, Williams RJ, Matthiessen P. The stress response of three-spined sticklebacks is modified in proportion to effluent exposure downstream of wastewater treatment works. Aquat Toxicol 2013; 126:382-92.
28. Gámez-Manrique W, Massago H, Abreu, Santos DJ, Criscuolo-Urbinati E. Respuesta del Piaractus mesopotamicus a estímulos de persecucián e hipoxia. Orinoquia 2009: 13(2):93-100.
29. Tort l. Stress and immune modulation in fish. Dev Comp Immunol 2011; 35(12):1366−1375.
30. Caruso G, Genovese I, Maricchiolo G, Modica A. Haematological, biochemical and immunological parameters as stress indicators in Dicentrarchus labrax and Sparus aurata farmed in off-shore cages. Aquac Int 2005; 13(1-2):67-73.
31. Pankhurst NW. The endocrinology of stress in fish: an environmental perspective. Gen Comp Endocr 2011; 170(2):265−275.