ORIGINAL
Monitoring rumen environment in finishing Lidia bulls
Monitorizacián del ambiente ruminal durante la fase de remate del toro de lidia
Juan García G,1* Ph.D, Raquel Posado F,1 Ph.D, José Zúñiga,2 Lic, María Tabernero de Paz R,1 Lic, Raúl Bodas R,1 Ph.D.
1Instituto Tecnolágico Agrario de Castilla y Leán - Subdireccián de Investigacián y Tecnología. Carretera de Carbajosa, s/n, Bajo. 37008 Salamanca. Spain.
2Garcisan Distribuciones, S.L. Bernardo Dorado, 2. 37008 Salamanca. Spain.
*Correspondence: gargarjj@itacyl.es
Received: September 2015; Accepted: January 2016..
ABSTRACT
Objective. The aim of this work was to characterize the changes in rumen pH and temperature in finishing Lidia breed bulls reared on pasture and fed a total mixed ration (TMR). Materials and methods. Five 4-year-old Lidia bulls received approximately 10 kg of the TMR per animal and day in the morning. Bulls could move freely in a 17-ha fenced area and express normally their feeding behaviour. Internal wireless boluses were used to collect pH and temperature values every 10 minutes throughout the measurement period. Results. Average daily pH was 6.2. Average and maximum daily temperatures were not high enough to be indicative of disease (infections of other pathologies). Conclusions. When rations and feeding systems are appropriately managed, Lidia bulls can be supplemented with concentrates in the finishing stages of their productive cycle without impairing the rumen environment.
Key words: Acidosis, pH, rations, temperature (Sources: AGROVOC).
RESUMEN
Objetivo. El presente trabajo pretendiá caracterizar las modificaciones que se producen en el pH y la temperatura ruminal de los toros de lidia, criados con un sistema de alimentacián basado en el suministro de una mezcla unifeed seca durante la etapa de acabado. Materiales y métodos. Se utilizaron 5 toros cuatreños de la raza de Lidia alimentados con, aproximadamente, 10 kg/animal y día de la mezcla unifeed a primera hora de la mañana. Los toros disponían de un espacio cercado de 17 ha, que les permitiría expresar sus patrones de comportamiento de pastoreo en libertad con plena normalidad. El pH y la temperatura ruminal se midiá de forma continua utilizando una sonda interna sin cables. Resultados. El pH medio fue de 6.20. Ni los valores de temperatura ruminal medios ni los máximos registrados son excesivamente altos como para ser indicativos del desarrollo de patologías o infecciones que pudieran afectar al estado de los animales. Conclusiones. Mediante el manejo adecuado de las raciones y del sistema de alimentacián, puede llevarse a cabo una suplementacián con alimentos concentrados para toros de lidia en la fase de remate de manera adecuada y respetuosa con su ambiente ruminal.
Palabras clave: Acidosis, pH, raciones, temperatura (Fuentes: AGROVOC).
INTRODUCTION
Amongst all the breeds of cattle, Lidia bulls are the only ones bred, raised and used with a productive purpose different from meat or milk: behavior.
Over the last decades, traditional extensive production systems of Lidia cattle have gradually been replaced by semi-intensive systems. Thus, the traditional extensive feeding system is followed by a fattening finishing period that usually begins in the summer-autumn of the year before the bullfight. During this finishing period the animals are kept in small fenced areas, where they are fed highly energetic and digestible rations (1,2). This excess of concentrates can cause ruminal acidosis, which has been related to falls during the bullfight, thus severely reducing animal performance (1,3).
Bartolomé (1) reported that 59% of fighting bulls had rumen pH values compatible with acidosis, 27% had liver damage, and 71% showed rumen paraqueratosis. This author linked liver damage to the animals falling during the bullfight and said that the number of falls was proportional to the severity of liver damage. He also considered that paraqueratosis and low rumen pH values could negatively influence the animal’s behavior while in the bullring.
Rumen temperature is a key factor in conditioning microbial growth, especially when rumen temperature sharply decreases, usually associated to cold water or forage intake (4). The range of physiological rumen temperature (38-42°C) varies depending on the authors (5,6).
It is, therefore, necessary to deepen the study of this disease and the influence of current feeding systems on bullfight performance with the goal of achieving the required animal volume and fine appearance without compromising rumen function. Thus, the aim of the present work was to study rumen characteristics of finishing bulls fed a total mixed ration.
MATERIALS AND METHODS
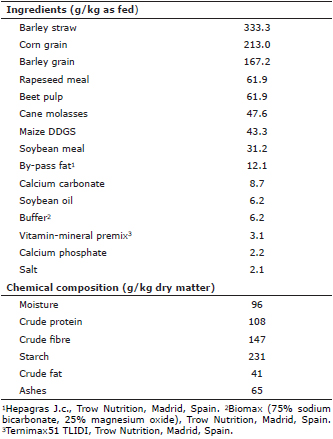
Animals and diets. Five 4-year-old Lidia bulls were used. Animals were handled according to the usual management in their farm, (Tejeda y Segoyuela (Salamanca, Spain; 40° 37’ 56" N 6° 01´ 21" O; 927 m above sea level, Köppen-Geiger”s climate classification: Csb), and were fed on a total mixed ration (TMR) whose ingredients and chemical composition are shown in table 1. On average, animals received 10 kg per day of the TMR, which was supplied early in the morning once a day by means of a feed mixer wagon.
Table 1. Ingredients and chemical composition of the total mixed ration (TMR).
Animal handling and management was conducted according to Directive 2010/63/EU for the protection of animals used for scientific purposes (7) and, albeit the experimental conditions did not entail animal suffering, the approval certification from the Instituto Tecnolágico Agrario de Castilla y Leán Ethical Committee for Animal Research was gathered. Animals were able to freely and normally move, graze and behave in a fenced space of 17 hectares.
Monitoring of rumen pH and temperature. Internal wireless smaXtec® boluses (smaXtec animal care sales GMBH, Graz, Austria) were used for collecting rumen pH and temperature data every 10 min. Each bolus ended up in the reticulum (where it remained until the animal was slaughtered) after being calibrated (pH 4 and 7) and introduced using an oral balling gun, following the manufacturer instructions. Data were recorded for a period of 37 (±8.2) days.
Analytical procedures. Procedures described by AOAC (8) were used to determine dry matter (DM, AOAC official method 934.01), ash (AOAC official method 942.05), and Kjeldahl N (AOAC official method 976.06). Neutral-detergent fibre (NDF, expressed including the residual ash) was determined by the method of Van Soest et al (9), adding sodium sulphite to the solution.
The chemical composition and concentrate and mixed ration was analyzed by Master Lab España Analytical Services (Tres Cantos, Madrid, Spain).
Statistical analysis. Data on rumen pH and temperature were first averaged for each day: maximum, minimum and mean, area under the curve, and time spent below pH thresholds of 7.0, 6.6, 6.2, 5.8, 5.4 and 5.0. The area under the curve was calculated by multiplying the absolute value of the deviation in pH by the time (min) the pH was below the threshold, and expressed as pH units×min. The value of the average pH for each hour within each day was also calculated for each animal.
Temperature data were summarized as maximum, minimum, and mean and °C. The area under the curve was calculated as indicated for pH. Temperature data for times of water intake of each animal were identified: the temperature shows a deep decrease followed by a slow increase to near temperature pre-water intake (10). The start of a drinking event was identified when rumen temperature suffered a decline greater than 0.28°C since the previous measurement. A drinking event ended when temperature was above 38.4°C or when temperature stopped increasing during a period of 10 min (10). The correlation analysis between rumen pH and temperature was performed using SPSS 16.0 for Windows (IBM Corp., New York, USA).
RESULTS
Descriptive statistics of rumen pH values are given in table 2. Average rumen pH was around 6.20, which can be considered as physiologically normal.
Table 2. Mean values of daily rumen pH in finishing Lidia bulls.
Maximum and minimum values of hours at maximum and minimum pH seemed to indicate that these pH values were reached sometimes at specific moments during the night. Nevertheless, quartiles, mode and mean values indicate that minimum pH was often reached in the afternoon (between 15:00 and 17:00), that is to say, 5 to 8 hours after feed supply. Likewise, maximum pH value was reached early in the morning (between 8:00 and 10:00). The area under the curve (Time (min/day) x pH) for pH below 5.4 was, on average, 14 min, with a maximum of 30 min for some animals.
Table 3 shows average temperature values of the 5 animals monitored in the experiment. Neither average nor maximum values observed in this experiment were high enough to be related to the development of pathologies or infections that could affect the animals’ health. The relative low values of minimum ruminal tempetature (around 32°C) observed could be linked to the moments when the animals drank water.
Table 3. Average daily values of rumen temperature (T, °C) in finishing Lidia bulls.
DISCUSSION
The pH values observed in this experiment were close to those reported by Bodas et al (11) and above those indicated by other authors (1,11,12) for animals of similar characteristics.
Maximum value of pH is reached just before feed supply, which agrees with the findings reported by Calsamiglia et al (13) and González et al (14). Feed intake takes place around maximum pH values, whilst minimum pH values were the result of the maximum fermentative activity within the rumen after a considerable amount of feed intake. According to Crater et al (15), pH value gradually drops immediately after feed supply and returns to previous values in 24 h, as it can be seen in figures 1 and 2.
Figure 1. Actual measurements of rumen pH and temperature for three consecutive days in one Lidia bull fed on a total mixed ration.
Figure 2. Evolution of rumen pH throughout the day (average of all the days) for each bull and average of the 5 bulls.
The average pH value observed in this experiment was 6.22, slightly higher than the 6.08 reported by Bartolomé (1), which can be considered as indicative of chronic acidosis (14). It is well known that when pH drops beyond this value, fibre digestibility decreases (6). Bartolomé (1) also found evident symptoms of acute ruminal acidosis in all Lidia bulls studied at the end of their productive cycle. This discrepancy between our results and those of former studies could be essentially due to two factors. The first one is related to the way the samples were taken: whereas in our study rumen conditions were continuously monitored, in Bartolomé’s (1) study rumen pH was only measured once the animal had been slaughtered, that is to say, after the animal had suffered acute stress and dehydration. On the other hand, feeding management instead of feed composition (13) is the decisive factor determining rumen pH and the consequent risk of rumen acidosis. Thus, albeit feed was distributed to ensure a minimum TMR intake of 10 kg per animal, the management system in a fenced area of 17 hectares allowed the animals to graze freely.
Notwithstanding, while the animals graze on a large area, they are likely to spend most of their time away from the spots where TMR is supplied. This circumstance together with the fact that TMR was supplied to the animals in the early morning and remained available in the feeder all day long may contribute to differences in daily feed intake pattern among animals. Hence, when feeds are distributed in punctual moments, even though the space in the feeder is large enough for all the animals, competitive social conditions can lead to competition between individuals, thus reducing the number of meals per day and increasing the amount of feed ingested at each meal, therefore deregulating the mechanisms to maintain optimal rumen conditions (16,17). Each of the monitored animals showed a different daily rumen pH pattern (Figure 2), and there was no indication either through pH values or clinical signs that the animals were experiencing acidosis.
The pH value to ensure an optimum growth of ruminal microorganisms depends on the species of rumen microbiota considered; on average, this value is within the range of 5.5 and 6.9. When pH values are outside this range, the growth of lactobacilli is favored and acidosis ensues (5,18). The rumen pH values of the bulls could be considered physiological between 90 and 99% of the time depending on the animal, thus contributing to a normal ruminal function.
Daily variations in rumen pH could cause deep modifications in rumen microbiota, so that strong daily variations can be considered worse than a relatively low average rumen pH (12,13). Our results show that when the average time at pH under 5.4 are below 15 minutes, changes in ruminal microbiota are not likely to affect rumen physiology. Maintaining a relative low pH will foster the growth of clostridia and coliform bacteria that will provoke inflammation in the mucosa and the development of hyperkeratosis, thus acting as a barrier for volatile fatty acids absorption (5,6,19).
According to several authors (20-22), temperature is one of the key factors conditioning bacterial growth in the rumen. Rumen temperature is usually 1 or 2 °C above body temperature (i.e., between 38 and 42°C) due to the number of biochemical processes occurring in the rumen and the homoeothermic regulation of the animal. This temperature can be lowered by the intake of cold water or forage.
It was estimated that there were between 2 and 3 water intakes per day, the main of which took place around 14:00 h., presumably some time after the heaviest TMR intake (that probably took place around 10:00, Figure 2). According to the findings of Wright (23), grazing animals prefer to drink water several times during the day, alternating with feed intake, although water intake frequency highly depends on the relative distance between feed and water areas. Nevertheless, water intake also depends on other factors such as physiological condition, dry matter intake and feed composition, body size, production level, physical activity and environmental factors (e.g. temperature).
In the present study water intake was not related to a drop in ruminal pH because when temperature reached a value below the physiological level (30.9-33.2°C), pH values were within the physiological range (7,14). The amount of time the animals’ rumen presented high temperatures was relatively short: the higher the ruminal temperature, the lower the time spent at that values. Water temperature and water intake, together with the already mentioned factors, may increase the risk of suffering ruminal acidosis (13). Thus, when animals receive a ration that is likely to increase the risk of developing acidosis, drinking water immediately after feed intake will increase acid formation in the rumen (13).
Previous studies show that there is a negative relationship between ruminal pH and temperature during an acidosis episode, therefore, monitoring ruminal temperature could help to detect such episodes (24). Conversely to this suggestion, in the present study a significant positive relationship between daily average temperature and pH was observed, although it should be interpreted with caution, given the low value of the correlation coefficient (r=0.135; p=0.03; Figure 3).
Figure 3. Relationship between daily average temperature and pH of the 5 bulls (r=0.153; p=0.03).
The results recorded in this work show that average the ruminal pH in finishing Lidia bulls fed on a TMR was within the range regarded as physiological. As for rumen temperature, neither average nor maximum values were too high to affect the animals. Consequentially, when feeding system is conveniently managed, supplementing finishing Lidia bulls with concentrate rations does not adversely affect the ruminal environment.
Acknowledgements
Funds received from Garcisan Distribuciones S.L. by means of the project entitled ‘Monitorizacián del ambiente ruminal durante la fase de remate del toro de lidia’ (Monitoring ruminal environment in finishing Lidia bulls).
REFERENCES
1. Bartolomé DJ. Influencia de la acidosis ruminal en el síndrome de caída y la respuesta etolágica del toro de lidia en la plaza [Tesis Doctoral]. Leán (Spain): Universidad de Leán; 2009.
2. Jordán D, Villa NA, Gutiérrez M, Gallego ÁB, Ochoa GA, Ceballos A. Blood chemistry in bullfighting cattle maintained under grazing conditions in Andes Mountains from Colombia. Rev Colomb Ciencias Pecu 2006; 19(1):18−26.
3. Lomillos Pérez JM, Alonso de la Varga M, Gaudioso Lacasa V. Análisis de la evolucián del manejo en las explotaciones de toro de lidia: desafíos del sector. ITEA-Informacián Técnica Econámica Agraria 2013; 109:49-68.
4. Crater AR, Barboza PS, Forster RJ. Regulation of rumen fermentation during seasonal fluctuations in food intake of muskoxen. Comp Biochem Physiol Part A Mol Integr Physiol 2007; 146(2):233−241.
5. Nagaraja TG, Titgemeyer EC. Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook. J Dairy Sci 2007; 90(Suppl 1):E17−E38.
6. Plaizier JC, Krause DO, Gozho GN, McBride BW. Subacute ruminal acidosis in dairy cows: the physiological causes, incidence and consequences. Vet J 2008; 176(1):21−31.
7. Directiva 2010/63/UE del Parlamento Europeo y del Consejo de 22 de septiembre de 2010 relativa a la proteccián de los animales utilizados para fines científicos. Diario Oficial de la Unián Europea, 20 de octubre de 2010; L276: 33−79.
8. AOAC. Official Methods of Analysis. 19th ed. Gaithersburg, MD: Association of Analitycal Chemists; 2012.
9. Van Soest PJ, Robertson JB, Lewis BA. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J Dairy Sci 1991; 74(10):3583−3597.
10. Dye TK, Richards CJ. Effect of water consumption on rumen temperature. J Anim Sci. 2008; 86 (Suppl 3): 114.
11. Bodas R, Posado R, Bartolomé DJ, Tabernero de Paz MJ, Herráiz P, Rebollo E, et al. Ruminal pH and temperature, papilla characteristics, and animal performance of fattening calves fed concentrate or maize silage-based diets. Chil J Agric Res 2014; 74(3):280−285.
12. Moya D, Mazzenga A, Holtshausen L, Cozzi G, González LA, Calsamiglia S, et al. Feeding behavior and ruminal acidosis in beef cattle offered a total mixed ration or dietary components separately. J Anim Sci 2011; 89(2):520−530.
13. Calsamiglia S, Cardozo PW, Ferret A, Bach A. Changes in rumen microbial fermentation are due to a combined effect of type of diet and pH. J Anim Sci 2007; 86(3):702−711.
14. González LA, Manteca X, Calsamiglia S, Schwartzkopf-Genswein KS, Ferret A. Ruminal acidosis in feedlot cattle: Interplay between feed ingredients, rumen function and feeding behavior (a review). Anim Feed Sci Technol 2012; 172(1-2):66−79.
15. Crater AR, Barboza PS. The Rumen in Winter: Cold Shocks in Naturally Feeding Muskoxen (Ovibos moschatus). J Mammal 2007; 8(3):625−631.
16. Schwartzkopf-Genswein KS, Beauchemin KA, McAllister TA, Gibb DJ, Streeter M, Kennedy AD. Effect of feed delivery fluctuations and feeding time on ruminal acidosis, growth performance, and feeding behavior of feedlot cattle. J Anim Sci 2004; 82(11):3357−3365.
17. González LA, Ferret A, Manteca X, Ruíz-de-la-Torre JL, Calsamiglia S, Devant M, et al. Performance, behavior, and welfare of Friesian heifers housed in pens with two, four, and eight individuals per concentrate feeding place. J Anim Sci 2008; 86(6):1446−1458.
18. Brown MS, Ponce CH, Pulikanti R. Adaptation of beef cattle to high-concentrate diets: Performance and ruminal metabolism. J Anim Sci 2006; 84(13 Suppl):E25−E33.
19. Penner GB, Taniguchi M, Guan LL, Beauchemin KA, Oba M. Effect of dietary forage to concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue. J Dairy Sci 2009; 92 (6): 2767−2781.
20. AlZahal O, Kebreab E, France J, Froetschel M, McBride BW. Ruminal temperature may aid in the detection of subacute ruminal acidosis. J Dairy Sci 2008; 91(1):202−207.
21. AlZahal O, Rustomo B, Odongo NE, Duffield TF, McBride BW. Technical note: A system for continuous recording of ruminal pH in cattle. J Anim Sci 2007; 85(1):213−217.
22. AlZahal O, Steele MA, Valdes EV, McBride BW. Technical note: The use of a telemetric system to continuously monitor ruminal temperature and to predict ruminal pH in cattle. J Dairy Sci 2009; 92(11):5697−5701.
23. Wright CL. Management of water quality for beef cattle. Vet Clin North Am Food Anim Pract 2007; 23(1):91−103.
24. Wahrmund JL, Ronchesel JR, Krehbiel CR, Goad CL, Trost SM, Richards CJ. Ruminal acidosis challenge impact on ruminal temperature in feedlot cattle. J Anim Sci 2012; 90(8):2794−2801.