Caracterización anatomopatológica de neumonías en cuyes producidas por Streptococcus pneumoniae en crianza intensiva
Anatomopathological characterization of pneumonia in guinea pigs caused by Streptococcus pneumoniae in intensive breeding

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Mostrar biografía de los autores
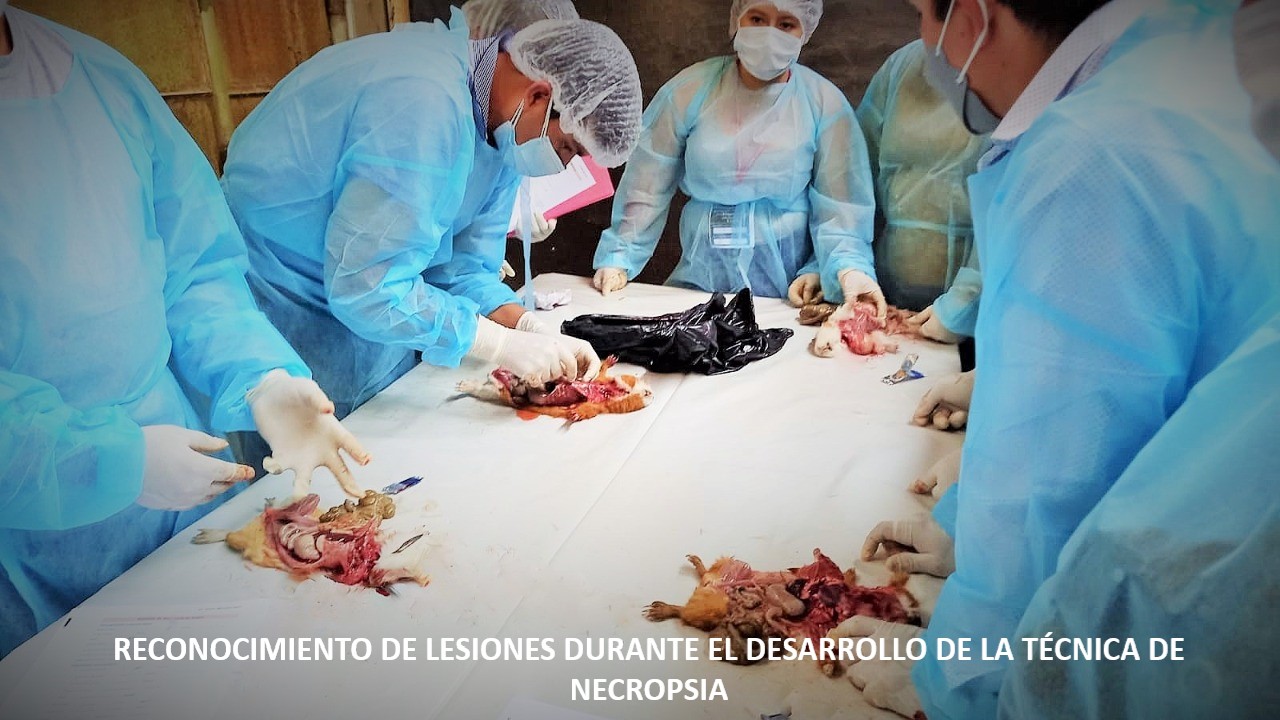
Objetivo. Caracterizar macroscópica y microscópicamente las lesiones causadas por Streptococcus pneumoniae en muestras de pulmón de cuyes (Cavia porcellus) de crianza intensiva de Lima. Materiales y métodos. Se hizo la necropsia de 138 cuyes con signos clínicos de neumonía, y se tomó la muestra de los pulmones afectados. Se realizó el aislamiento bacteriano para la identificación de Streptococcus pneumoniae, utilizando agar sangre y pruebas bioquímicas; se realizó el procesamiento histopatológico utilizando la coloración hematoxilina eosina (HE) de las muestras de tejidos conservados en formol al 10%. Resultados. Del total de los pulmones diagnosticados con neumonía en necropsia, el 17.4 % (24/138) resultó positivo a Streptococcus pneumoniae; De estos, el 75% (18/24) mostró características macroscópicas de neumonía intersticial/Neumonía broncointersticial y 25% (6/24) características macroscópicas de bronconeumonía. En la evaluación microscópica se encontró que el 37.5% presentó tanto para neumonía intersticial y neumonía broncointersticial, y un 25% de ellas corresponden a bronconeumonía. Conclusión. la neumonía intersticial, neumonía broncointersticial y bronconeumonía con diagnóstico de Streptococcus pneumoniae son patrones lesionales encontradas en sistemas de crianza intensiva de Lima.
Visitas del artículo 1414 | Visitas PDF
Descargas
- Tamura Y. Current Approach to Rodents as Patients. Journal of Exotic Pet Medicine. 2010; 19(1):36-55. https://doi.org/10.1053/j.jepm.2010.01.014
- Morales S. Patógenos oportunistas por transmisión fecal-oral en cuyes reproductores introducidos al distrito de San Marcos. Científica. 2012. 9(1):33-38. https://issuu.com/bibliotecacientifica/docs/cientifica-v9n1/37
- Minarikova A, Hauptman K, Jeklova E, Knotek Z, Jekl V. Diseases in pet guinea pigs: a retrospective study in 1000 animals. VetRecord. 2015; 177(8):1-10. http://dx.doi.org/10.1136/vr.103053
- Barthold SW, Griffey SM, Percy DH. Guinea Pig. Pathology of Laboratory Rodents and Rabbits. 4th. Ed. Chichester: John Wiley & Sons; 2016. http://doi.wiley.com/10.1002/9781118924051.ch05
- Roberts-Steel S, Oxley JA, Carroll A, Wills AP. Frequency of Owner-Reported Bacterial Infections in Pet Guinea Pigs. Animals. 2019; 9(9):649. http://dx.doi.org/10.3390/ani9090649
- Pittet LA, Hall-Stoodley L, Rutkowki MR, Harmese AG. Influenza Virus Infection Decreases Tracheal Mucociliary Velocity and Clearance of Streptococcus pneumoniae. Am J Respir Cell Mol Biol. 2010; 42(4):450-460. https://doi.org/10.1165/rcmb.2007-0417OC
- Zachary, JF. Respiratory System: Mediastinum and Pleurae. In: Missouri LS, Ed. Pathologic basis of veterinary disease. 6thed. Elsevier; 2017.
- Tilahun S, FekaduA, Mekibib B. Major Causes of Total Organ Condemnation and their Direct Financial Impact in Cattle Slaughtered at Hawassa Municipality Abattoir, Southern Ethiopia. J Vet Sci Technol. 2017; 8(5):1-5. https://doi.org/10.4172/2157-7579.1000473
- Opzeeland YPM. Welfare issues in causes of death in young pet rabbits. [Tesis Doctoral]. Utrecht: Faculty of Veterinary Medicine Theses, Universiteit Utrecht; 2012. https://dspace.library.uu.nl/handle/1874/225791
- Björklund C, Båge R, Morrell J, Kerstin de Verdier, Nisu L, Kjellinbro N et al. Diseases and causes of death among alpacas in Sweden: a retrospective study. Vet Rec. 2019; 6(1):1-7. http://dx.doi.org/10.1136/vetreco-2017-000239
- Oremaiyi T, Simon U, Emmanuel A, Samson A, Sunday I, Charles E, et al. Prevalence of Gender, Age and Breed in the Occurrence of Pneumonia in Goats Slaughtered At Gwagwalada Abattoir, Abuja. IOSR-JAVS. 2019; 12(9):72-77. https://www.iosrjournals.org/iosr-javs/papers/Vol12-issue9/Series-2/L1209027277.pdf
- Markey B, Leonard F, Archambault M, Cullinane A, Maguire D. Clinical Veterinary Microbiology. 2°ed. Canadá: Elsevier; 2013.
- Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comp Med; 2008; 58(4):324-340. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2706043/
- Witkowska A, Price J, Hughes C, Smith D, White K, Alibhai A, et al. The Effects of Diet on Anatomy, Physiology and Health in the Guinea Pig. J Anim Health Behav Sci. 2017; 1(1):103. https://www.hilarispublisher.com/open-access/the-effects-of-diet-on-anatomy-physiology-and-health-in-the-guinea-pig.pdf
- Gruszynski K, Young A, Levine SJ, Garvin JP, Brown S, Turner L, et al. Streptococcus equi subsp. zooepidemicus infections associated with guinea pigs. Emerg Infect Dis. 2015; 21(1):156-158. http://dx.doi.org/10.3201/eid2101.140640
- Kwon YK, Lipatov AS, Swayne DE. Bronchointerstitial Pneumonia in Guinea Pigs Following Inoculation with H5N1 High Pathogenicity Avian Influenza Virus. Vet Pathol. 2009; 46(1):138-141. https://dx.doi.org/10.1354/vp.46-1-138
- Hwang CK, Iuvone PM. Technical Brief: A comparison of two methods of euthanasia on retinal dopamine levels. Mol Vis . 2013; 19:1122–1124. http://www.molvis.org/molvis/v19/1122
- Roustan A, Perrin J, Berthelot-Ricou A, Lopez E, Botta A, Courbiere B. Evaluating methods of mouse euthanasia on the oocyte quality: cervical dislocation versus isoflurane inhalation. LAL. 2012; 46:167–169. https://doi.org/10.1258%2Fla.2012.011115
- Astaiza MJ, Benavides J, Chaves AC, Arciniegas MA, Quiroz HL. Estandarización de la técnica de necropsia en cuyes (cavia porcellus) en la Universidad de Nariño. REVIP. 2013; 2(2):77-83. http://revistas.udenar.edu.co/index.php/revip/article/view/448
- Van der Linden M, Al-Lahham A, Nicklas W, Reinert, RR. Molecular Characterization of Pneumococcal Isolates from Pets and Laboratory Animals. PLoS ONE. 2009; 4(12):1-9. https://doi.org/10.1371/journal.pone.0008286
- Lamm CG, Ferguson AC, Lehenbauer TW, Love BC. Streptococcal Infection in Dogs: A Retrospective Study of 393 Cases. Vet Pathol. 2010; 47(3):387-395 https://doi.org/10.1177/0300985809359601
- Yarto-Jaramillo E. Respiratory System Anatomy, Physiology, and Disease: Guinea Pigs and Chinchillas. Veterinary Clinics of North America: Exotic Animal Practice. 2011; 14(2):339-355. https://doi.org/10.1016/j.cvex.2011.03.008
- Rashid MM, Ferdoush MJ, Dipti M, Roy P, Rahman MM, Hossain MI, et al. Bacteriological and pathological investigation of goat lungs in Mymensingh and determination of antibiotic sensitivity. Bangl. J Vet Med. 2013; 11(2):159-166 https://doi.org/10.3329/bjvm.v11i2.19142
- Caicedo-Martínez JA, Ávila-Rubiano MA, Orellano-Badillo H, Sanjuanelo-Corredor DW. Patología pulmonar en ovinos faenados del norte del departamento de Bolívar, Colombia. Ciencia & Tecnología Agropecuaria. 2017; 18(3):555-569. https://doi.org/10.21930/rcta.vol18_num3_art:744
- Costa RA, Schild C, Silveira CS, Macías-Rioseco M, Mirazo S, Maya L, et al. Acute and chronic bovine pulmonary edema and emphysema in Uruguay. Pesq Veterinario Bras. 2018; 38(10):1929-1934. https://dx.doi.org/10.1590/1678-5150-pvb-5890
- Cross RW, Fenton KA, Geisbert JB, Mire CE, Geisbert TW. Modeling the Disease Course of Zaire ebolavirus Infection in the Outbred Guinea Pig. J Infect Dis. 2015; 212(2):305-315. https://doi.org/10.1093/infdis/jiv237
- Junga k, Lee CS, Kang BK, Park BK, Oh JS, Song DS. Pathology in dogs with experimental canine H3N2 influenza virus infection. Res Vet Sci. 2010; 88(3):523-527. https://doi.org/10.1016/j.rvsc.2009.11.007
- Hong-Fei S, Yuan-Mao Z, Xiu-Mei D, Hong C, Lei M, Shu W, et al. Pathogenesis of a genotype C strain of bovine parainfluenza virus type 3 infection in albino guinea pigs. Virus Res. 2014; 188:1-7. https://doi.org/10.1016/j.virusres.2014.03.017
- Bell TM, Bunton TE, Shaia CI, Raymond JW, Honnold SP, Donnelly GC, et al. Pathogenesis of Bolivian Hemorrhagic Fever in Guinea Pigs. Vet Pathol. 2015; 53(1):190-199. https://doi.org/10.1177/0300985815588609
- Tyagita H, Bahaman AR, Jasni S, Ibrahim TAT, Fuzina NH. Pathogenicity of Leptospira icterohaemorrhagiae serovar Lai strain Langkawi in guinea pigs (Cavia porcellus). J Vet Malaysia. 2019; 31(1):1-11. http://psasir.upm.edu.my/id/eprint/70119/1/70119.pdf
- Vallejo DA, Chaves CA, Morillo DP, Astaíza JM, Melo CC. Determinación histopatológica de patrones neumónicos del complejo respiratorio bovino en el municipio Pasto, Colombia. Ces Med Vet Zootec. 2016; 11(1):88-99. http://revistas.ces.edu.co/index.php/mvz/article/view/3822/2536
- Betancur-Hurtado C, Castañeda-Ternera J, Gonzáles-Tous M. Inmunopatología del complejo respiratorio bovino en terneros neonatos en Montera-Colombia. Revista Científica. 2017; 27(2):95–102. https://www.redalyc.org/jatsRepo/959/95951040005/html/index.html
- Yershov LA, Jordan BS, Guymon CH, Dubick MA. Relationship beteween the inoculum dose Streptococcus pneumoniae and pneumonia onset in a rabbit model. Eur Respir J. 2005; 25(4):693-700. https://doi.org/10.1183/09031936.05.00091904
- Guzmán H, Mogollón JD, Rincón MA, Lora AM. Correlación entre las lesiones macroscópicas e histopatológicas de la neumonía enzoótica y la detección del Mycoplasma hyopneumoniae por PCR anidada en lavados bronco alveolares en cerdos al sacrificio. Rev Med Vet Zoot. 2008; 55(1):39-48. https://revistas.unal.edu.co/index.php/remevez/article/view/10536/14308
- Song J, Wang G, Hoenerhoff MJ, Ruan J, Yang D, Zhang J, et al. Bacterial and Pneumocystis Infections in the Lungs of Gene-Knockout Rabbits with Severe Combined Immunodeficiency. Front. Immunol. 2018; 9(429):1-9. https://doi.org/10.3389/fimmu.2018.00429
- Ballinger MN, Standiford TJ. Postinfluenza Bacterial Pneumonia: Host Defenses Gone Awry. JICR. 2010; 30(9):643–652. https://doi.org/10.1089/jir.2010.0049
- Hick PM, Read AJ, Lugton I, Busfield F, Dawood KE, Gabor L, et al. Coronavirus infection in intensively managed cattle with respiratory disease. Aust Vet J. 2012; 90(10):381-386 https://doi.org/10.1111/j.1751-0813.2012.00978.x
- Jong-Hwan P, Seung-Hyeok S, Min-Won B, Hui-Young L, Dong-Jae K, Jung-Sik C, et al. Microbiological Monitoring of Guinea Pigs Reared Conventionally at Two Breeding Facilities in Korea. Exp Anim. 2006; 55(5):427-432. https://doi.org/10.1538/expanim.55.427
- Campos ALB, Silva LE, Souza T, Gabardo MP, Valério de Carvalho O, Costa M, et al. Verification of natural infection of peridomestic rodents by PCV2 on commercial swine farms. Res Vet Sci. 2013; 94(3):764–768. http://dx.doi.org/10.1016/j.rvsc.2012.10.006
- Bell TM, Shaia CI, Bearss JJ, Mattix ME, Koistinen KA, Honnold S. P, et al. Temporal progression of lesions in guinea pigs infected with Lassa virus. Vet Pathol. 2017; 54(3):549-562. https://doi.org/10.1177/0300985816677153























