Investigación de la efectividad de varios métodos de tratamiento en cabras con papilomatosis cutánea
The Investigation of the effectiveness of various treatment methods in goats with cutaneous papillomatosis

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Mostrar biografía de los autores
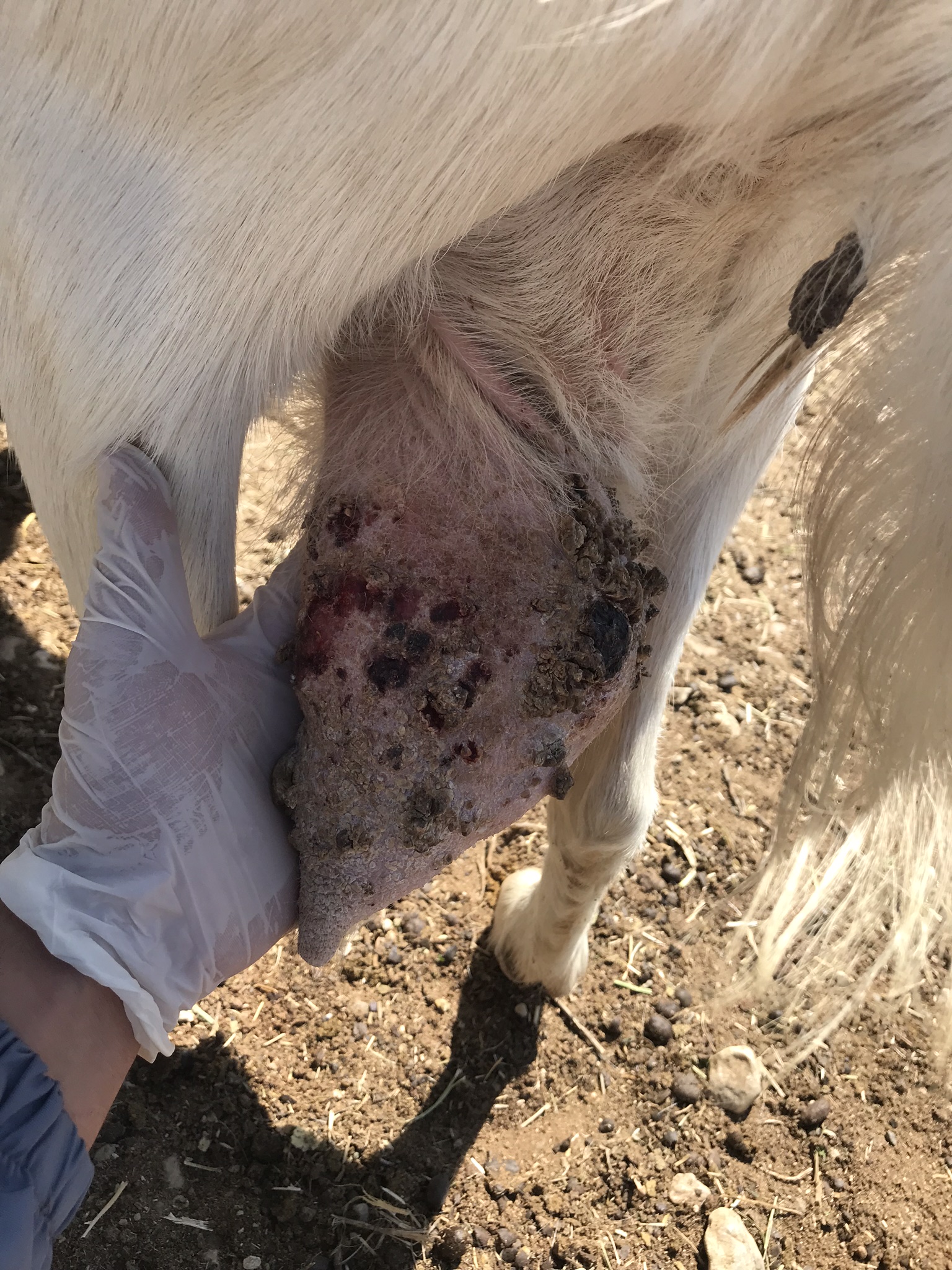
Objetivo. Este estudio investigó los efectos de recuperación del levamisol, el extracto de Tarantula cubensis y la combinación de levamisol+extracto de T. cubensis en cabras con papilomatosis cutánea. Materiales y métodos. Se incluyeron en el estudio cuarenta cabras de pelo entre 2 y 4 años de edad en período de lactancia, que fueron diagnosticadas clínicamente de papilomatosis, y se dividieron en cuatro grupos aleatoriamente, cada uno con diez cabras. No se realizó ninguna aplicación en cabras en el grupo de control. Se administraron levamisol (5mg/kg, por vía intramuscular), extracto de T.cubensis (4ml, por vía subcutánea) y levamisol (5mg/kg, por vía intramuscular) y extracto de T.cubensis (4ml, por vía subcutánea) a las cabras en el grupo de levamisol, grupo de extracto de T. cubensis y grupo combinado, respectivamente, dos veces a intervalos semanales. Todos los animales de los grupos se controlaron a intervalos de 15 días durante tres meses. Resultados. Si bien no se observó recuperación en el grupo de control, se observó regresión en solo el papiloma de un animal (10%). Las tasas de recuperación y regresión fueron 40% y 40% en el grupo de levamisol, 30% y 40% en el grupo de extracto de T. cubensis y 60% y 30% en el grupo combinado, respectivamente. Conclusiones. Se encontró que los tres tratamientos aplicados en cabras para tratar la papilomatosis cutánea eran efectivos en diferentes grados. Cuando se comparó entre los grupos de tratamiento, se encontró que la combinación de extracto de levamisol+T. cubensis era un tratamiento más efectivo.
Visitas del artículo 687 | Visitas PDF
Descargas
- King AMQ, Adams MJ, Carsten EB, Lefkowitz EJ. Virus taxonomy: Classification and nomenclature of viruses. Ninth Report of the International Committee on Taxonomy of Viruses, Elsevier Inc. 2012.
- Van Doorslaer K. Evolution of the Papillomaviridae. Virol. 2013; 445(1-2):11-20. https://doi.org/10.1016/j.virol.2013.05.012
- Araldi RP, Assaf SMR, De Carvalho RF, Caldas MA, De Carvalho R, De Souza JM, Magnelli RF, Modolo DG, Roperto FP, Stocco RC, Beçak W. Papillomaviruses: a systematic review. Genet Mol Biol 2017; 40:1-21. https://doi.org/10.1590/1678-4685-gmb-2016-0128
- Araldi RP, Melo TC, Neves AC, Spadacci-Morena DD, Magnelli RF, Modolo DG, De Sa Junior PL, Mazucchelli-de-Souza J, Carvalho RF, Beçak W, Stocca RC. Hyperproliferative action of bovine papillomavirus: genetic and histopathological aspects. Genet Mol Res. 2015; 14(4):12942-12954. https://doi.org/10.4238/2015.October.21.15
- Rector A, Van Ranst M. Animal papillomaviruses. Virol. 2013; 445(1-2):213-223. https://doi.org/10.1016/j.virol.2013.05.007
- Moulton JE. Cutaneous papillomas on the udders of milk goats. North American Veterinarian. 1954; 35:29–33.
- Van Doorslaer K, Rector A, Vos P, Van Ranst M. Genetic characterization of the capra hircus papillomavirus: A novel close-to-root artiodactyl papillomavirus. Virus Res. 2006; 118(1-2):164–169. https://doi.org/10.1016/j.virusres.2005.12.007
- Simeone P, Romanucci M, Rizzo C, Brandi R, Malatesta D, Di Guardo G, Bongiovanni L, Salda LD, Venuti A. Papillomaviruses in multiple tumours of twin goats. Open Vet. 2008; 2:33-36. https://doi.org/10.2174/1874318808002010033
- Dogan F, Dorttas SD, Dağalp SB, Ataseven VS, Alkan F. A teat papillomatosis case in a Damascus goat (Shami goat) in Hatay province, Turkey: a new putative papillomavirus? Arch Virol. 2018; 163(6):1635–1642. https://doi.org/10.1007/s00705-018-3781-2
- Lunardi M, Alfieri AA, Otonel RAA, De Alcantara BK, Rodrigues WB, De Miranda AB, and Alfieri AF. Genetic characterization of a novel bovine papillomavirus member of the Deltapapillomavirus genus. Vet Microbiol. 2013; 162(1):207–213. https://doi.org/10.1016/j.vetmic.2012.08.030
- Daudt C, Da Silva FRC, Lunardi M, Alves CBDT, Weber MN, Cibulski SP, Alfleri AF, Alfleri AA, Canal CW. Papillomaviruses in Ruminants: An update. Transbound Emerg Dis. 2018; 65(5):1381-1395. https://doi.org/10.1111/tbed.12868
- Maclachlan NJ, Dubovi EJ. Papillomaviridae and Polyomaviridae. In: Fenner’s Veterinary Virology. 5th ed, Academic Press, London. 2016. https://doi.org/10.1016/B978-0-12-800946-8.00011-8
- Tan MT, Yıldırım Y, Sözmen M, Dağalp SB, Yılmaz V, Kırmızıgül AH, and Gökçe E. A histopathological, immunohistochemical and molecular study of cutaneous bovine papillomatosis. Kafkas Univ Vet Fak Derg. 2012; 18:739-744. https://doi.org/ 10.9775/kvfd.2012.5341
- Ugochukwu ICI, Aneke CI, Idoko IS, Sani NA, Amoche AJ, Mshiela WP, Ede RE, İbrahim NDG, Njoku CIO, Sackey AKB. Bovine papilloma: aetiology, pathology, immunology, disease status, diagnosis, control, prevention and treatment: a review. Comp Clin Path. 2019; 28:737–745. https://doi.org/10.1007/s00580-018-2785-3
- Hamad MA, Al-Shammari AM, Odisho SM, Yaseen NY. Molecular and phylogenetic analysis of bovine papillomavirus type 1: first report in Iraqi cattle. Adv Virol. 2016; 1–7. https://doi.org/10.1155/2016/2143024
- Nasir L, Brandt S. Papillomavirus associated diseases of the horse. Vet Microbiol. 2013; 167(1-2):159–167. https://doi.org/10.1016/j.vetmic.2013.08.003
- Da Silva FRC, Daudt C, Streck AF, Weber MN, Filho RVL, Driemeier D, Canal CW. Genetic characterization of Amazonian bovine papillomavirus reveals the existence of four new putative types. Virus Gen. 2015; 51(1):77–84. https://doi.org/10.1007/s11262-015-1220-y
- Lunardi M, Tozato CC, Alfieri AF, De Alcantara BK, Vilas-Boas LA, Otonel RAA, Headley SA, Alfieri AA. Genetic diversity of bovine papillomavirus types, including two putative new types, in teat warts from dairy cattle herds. Arch Virol. 2016; 161(6):1569–1577. https://doi.org/10.1007/s00705-016-2820-0
- Roperto S, Russo V, Leonardi L, Martano M, Corrado F, Riccardi MG, Roperto F. Bovine papillomavirus type 13 expression in the urothelial bladder tumours of cattle. Transbound Emerg Dis. 2016; 63(6):628–634. https://doi.org/10.1111/tbed.12322
- Sharma S, Mahajan V, Gupta DK, Singh RS, Bansal BK. An epidemiological and pathological study of teat papillomas in caprines. Intas Polivet. 2015; 16:119-120. https://www.karger.com/INT
- Zhu W, Yuan D, Norimine J, Gao N, Fan S, Du Y, Li F, Yang M, Hu S, Dong J. Teat papillomatosis in dairy herds: First detection of bovine papillomavirus type 10 in China. J Vet Med Sci. 2019; 81(7):1063–1066. https://doi.org/10.1292/jvms.18-0449
- Munday JS. Bovine and human papillomaviruses: A comparative review. Vet Pathol. 2014; 51(6):1063–1075. https://doi.org/10.1177/0300985814537837
- Ranjan R, Ghumman SPS, Bhatt GR, Singh RS. Efficacy of autogenous vaccine and auto-hemotherapy in bovine cutaneous papillomatosis. Intas Polivet. 2013; 14:411-414. https://www.thefreelibrary.com/Efficacy+of+autogenous+vaccine+and+auto-hemotherapy+in+bovine...-a0375288607
- Çam Y, Kibar M, Atasever A, Atalay Ö, Beyaz L. Efficacy of levamisole and tarantula cubensis venom for the treatmentof bovine cutaneous papillomatosis. Vet Rec. 2007; 160(14):486-488. https://doi.org/10.1136/vr.160.14.486
- Zafar MA, Hasan M, Riaz A, Shamim A, Iqbal F, Yousaf A. Comparative therapeutic evaluation of partial excision of lesions and administering autogenous vaccine along with immune-modulators for the treatment of bovine papillomatosis. Vet Scie Res Review. 2015; 1:6-9. http://dx.doi.org/10.17582/journal.vsrr/2015.1.1.6.9
- Vijayasarathi MK, Shebaa A, Venkatesan M. Therapeutic management of bovine calf papillomatosis. Indian Vet. 2018; 95(09):60-61. http://ivj.org.in/users/members/viewa
- Kale M, Saltık HS, Hasırcıoğlu S, Yıldırım Y, Yavru S, Mamak N, Atlı K. Treatment of bovine papillomavirus-induced teat warts in a cow by using podophyllin magistral formula and autologous vaccine applications together. Indian J Anim Res. 2019; 53:832-836. https://doi.org/10.18805/ijar.B-911
- Renoux G. The general immunopharmocology of levamisole. Drugs. 1980; 20(2):89-99. https://doi.org/10.2165/00003495-198020020-00001
- Tekelioğlu BK, Parkan C, Yılmaz A, Turan N, Gürel A, Berber K, et al. Canine papillomatosis: Clinical outcome after oral isotherapy, subcutaneous tarantula cubensis venom and oral levamisole-azithromycin treatment. Bulg J Vet Med. 2017; 20(Suppl. 1):248-253. http://uni-sz.bg/truni6/wp-content/uploads/vmf/file/43%20Tekelioglu+.pdf
- Dhule SJ. Auto-Haemotherapy for management of caprine papillomatosis. Intas Polivet. 2013; 14(2):425-426. https://www.cabdirect.org/cabdirect/FullTextPDF/2014/20143138960.pdf
- İçen H, Sekin S, Simsek A, Kochan A, Tunik S. The efficacy of tarantula cubensis extract (theranekron) in treatment of canine oral papillomatosis. AJAVA. 2011; 6(7):744-749. https://scialert.net/abstract/?doi=ajava.2011.744.749
- Gültiken N, Vural RM. The effect of tarantula cubensis extract applied in pre and postoperative period of canine mammary tumours. JIVS. 2007; 2:13-23. https://dergipark.org.tr/tr/download/article-file/268754
- Paksoy Z, Gulesci N, Kandemir FM, Dinçel GD. Effectiveness of levamisole and tarantula cubensis extract in the treatment of teat papillomatosis of cows. Indian J Anim Res. 2015; 49(5):704-708. https://doi.org/10.18805/ijar.5586
- McDougall S, Murdough P, Pankey W, Delaney C, Barlow J, Scruton D. Relationships among somatic cell count, California mastitis test, impedance and bacteriological status of milk in goats and sheep in early lactation. Small Ruminant Res. 2001; 40(3):245–254. https://doi.org/10.1016/S0921-4488(01)00185-7























