Investigación de parámetros bioquímicos y perfil de citocinas en ovejas con ectima contagioso
Investigation of biochemical parameters and cytokine profile in sheep with contagious ecthyma inmunología del ectima infeccioso

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Mostrar biografía de los autores
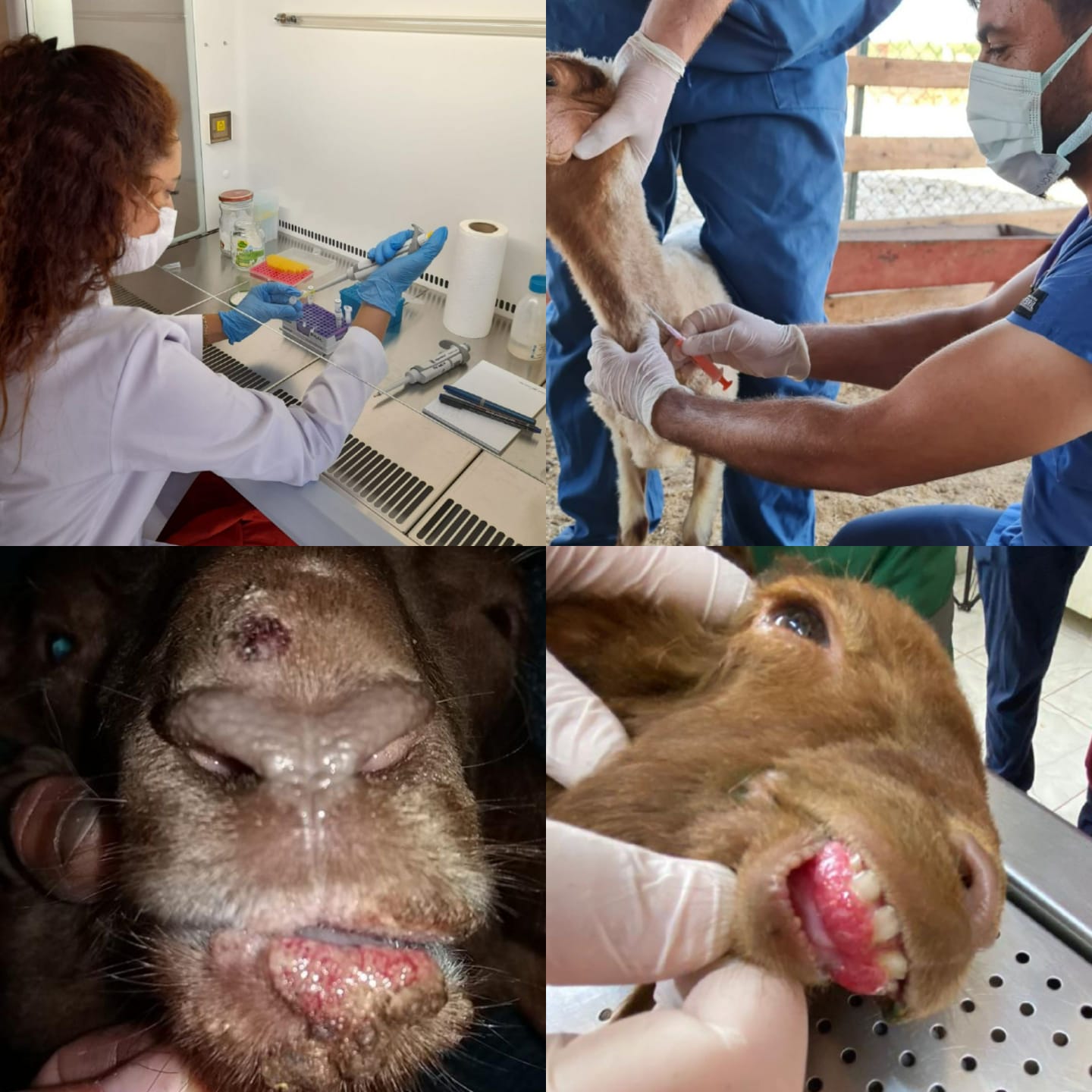
Objetivo. El ectima contagioso es una enfermedad zoonótica mundial con una amplia gama de hospedadores y una alta morbilidad que causa graves pérdidas económicas. Materiales y métodos. Se utilizaron 28 ovejas no vacunadas de 1 a 4 meses que presentaban síntomas clínicos de ectima y 10 ovejas sanas del mismo rango de edad. Se tomaron medidas de ALB, ALT, BUN, CHOL, CRE, GGT, GLU, TBIL y TP. Las costras obtenidas se homogeneizaron antes de realizar la extracción y PCR. Se usó ELISA para determinar los niveles de IL-2 e IL-4 en el suero animal positivo a PCR. Resultados. El análisis bioquímico reveló que los valores de ALT, BUN, GGT y CRE en los animales infectados fueron significativamente más altos que en el grupo de control (p = 0,000 yp = 0,001) mientras que los valores de TP y GLU fueron significativamente más bajos (p = 0,000). No hubo diferencias significativas en los valores de ALB, CHOL y TBIL (p = 0,1, p = 0,05, p = 0,08). En cuanto al perfil inmune, los animales infectados tenían niveles de IL-2 (28%) e IL-4 (60%) significativamente más altos que el grupo de control (p = 0,008 yp = 0,001). Conclusión. Los hallazgos indican que las citocinas Th1 (IL-2) y Th2 (IL-4) coexisten mientras que la respuesta de citocinas dominante en los animales infectados es Th2. Se cree que el resultado nos ayudará a comprender mejor la patogenia y las opciones de tratamiento de la enfermedad.
Visitas del artículo 526 | Visitas PDF
Descargas
- Karki M, Venkatesan G, Kumar A, Kumar S, Bora DP. Contagious ecthyma of sheep and goats: A comprehensive review on epidemiology, immunity, diagnostics and control measures. Veterinarski Arhiv. 2019; 89(3):393-423. https://doi.org/10.24099/vet.arhiv.0208
- Bala JA, Balakrishnan KN, Jesse FFA, Abdullah AA, bin Noorzahari, MS, Ghazali MT, et al. Identification of strain diversity and phylogenetic analysis based on two major essential proteins of Orf viruses isolated from several clinical cases reported in Malaysia. Infect Genet Evol. 2020; 77:104076. https://doi.org/10.1016/j.meegid.2019.104076
- Rintoul JL, Lemay CG, Tai LH, Stanford MM, Falls TJ, De Souza CT, et al. ORFV: a novel oncolytic and immune stimulating parapoxvirus therapeutic. Mol Ther. 2012; 20(6):1148-1157. https://doi.org/10.1038/mt.2011.301
- Yang Y, Qin X, Wang G, Jin J, Shang Y, Zhang Z. Development of an isothermoal amplification-based assay for rapid visual detection of an Orf virus. Virol J. 2016; 13(1):1-6. https://doi.org/10.1186/s12985-016-0502-x
- Nandi S, De UK, Chowdhury S. Current status of contagious ecthyma or orf disease in goat and sheep—a global perspective. Small Rumin Res. 2011; 96(2-3):73-82. https://doi.org/10.1016/j.smallrumres.2010.11.018
- Khalafalla AI, Elhag AE, Ishag HZA. Field investigation and phylogenetic characterization of orf virus (ORFV) circulating in small ruminants and Pseudocowpoxvirus (PCPV) in dromedary camels of eastern Sudan. Heliyon. 2020; 6(3):e03595. https://doi.org/10.1016/j.heliyon.2020.e03595
- Bergqvist C, Kurban M, Abbas O. Orf virus infection. Rev Med Virol. 2017; 27(4):e1932. https://doi.org/10.1002/rmv.1932
- Lawan Z, Bala JA, Bukar AM, Balakrishnan KN, Mangga HK, Abdullah FFJ, et al. Contagious ecthyma: how serious is the disease worldwide? Anim Health Res Rev. 2021; 22(1):1-16. https://doi.org/10.1017/S1466252320000018
- Khatiwada S, Delhon G, Nagendraprabhu P, Chaulagain S, Luo S, Diel DG, et al. A parapoxviral virion protein inhibits NF-κB signaling early in infection. PLoS Pathog. 2017; 13(8):e1006561. https://doi.org/10.1371/journal.ppat.1006561
- Quaresma JAS. Organization of the skin immune system and compartmentalized immune responses in infectious diseases. Clin Microbiol Rev. 2019; 32(4):e00034-18. https://doi.org/10.1128/CMR.00034-18
- Muhsen M, Protschka M, Schneider LE, Müller U, Köhler G, Magin TM, et al. Orf virus (ORFV) infection in a three-dimensional human skin model: Characteristic cellular alterations and interference with keratinocyte differentiation. Plos One. 2019; 14(1):e0210504. https://doi.org/10.1371/journal.pone.0210504
- Dalal A, Kumar V, Chaudhary D, Bansal N, Kumar A, Kakker N, et al. Past and Present Overview of “Orf”. Int J Curr Microbiol App Sci. 2017; 6(12):2159-2173. https://doi.org/10.20546/ijcmas.2017.612.248
- Haıg DM, Mcınnes C, Deane D, Reıd H, Mercer AA. The immune and inflammatory response to orf virus. Comp Immunol Microbiol. 1997; 20(3):197-204. https://doi.org/10.1016/S0147-9571(96)00045-8
- Fatehi F, Kyrychko YN, Molchanov R, Blyuss KB. Bifurcations and multistability in a model of cytokine-mediated autoimmunity. Int. J. Bifurc Chaos Appl Sci Eng. 2019; 29(03):1950034. https://doi.org/10.1142/S0218127419500342
- Strugaru OR, Velescu E, Perianu T, Bocăneţi F, Scagliarini A. Detection of orf virus and papillomavirus out of samples from goats and cattle which were gathered by multiplex PCR Romania. Cercet agron Mold. 2012; 45(2):85-88. http://www.uaiasi.ro/CERCET_AGROMOLD/CA2-12-10.pdf
- Zhao K, He W, Gao W, Lu H, Han T, Li J, et al. Orf virus DNA vaccines expressing ORFV 011 and ORFV 059 chimeric protein enhances immunogenicity. Virol J. 2011; 8(1):1-12. https://virologyj.biomedcentral.com/track/pdf/10.1186/1743-422X-8-562.pdf
- Asl SAK, Aslani MR, Mohebbi A, Mokhtari A. Hematological and biochemical evaluation of goats naturally infected with contagious ecthyma. Hematol. 2019; 10(2):43-47. https://doi.org/10.22067/veterinary.v2i10.69865
- Kataria AK, Kataria N, Gahlot AK. Large scale outbreaks of peste des petits ruminants in sheep and goats in Thar desert of India. Slov Vet Res. 2007; 44(4):123-132. http://www2.vf.uni-lj.si/ZB/SlovVetRes_44_(4)_pp123-132.pdf
- Narnaware SD, Nagarajan G, Dahiya SS. Hemato-biochemical studies in Indian camel (Camelus dromedarius) affected with contagious ecthyma. Indian J Appl Res. 2015; 39(2):168-170. https://doi.org/10.5958/0973-970X.2015.00038.3
- Gameel AA, Abu Elzein EME, Housawi FMT, Agib A, Ibrahim AO. Naturally occurring contagious ecthyma in lambs in Saudi Arabia. Rev Elev Med Vet Pays Trop. 1995; 48(3):233-235. https://europepmc.org/article/med/8745744
- Cantorna MT, Snyder L, Lin YD, Yang L. Vitamin D and 1, 25 (OH) 2D regulation of T cells. Nutrients. 2015; 7(4):3011-3021. https://doi.org/10.3390/nu7043011
- Wu Y, Tian Z, Wei H. Developmental and functional control of natural killer cells by cytokines. Front Immunol. 2017; 8:930. https://doi.org/10.3389/fimmu.2017.00930
- Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012; 12(2):136-148. https://doi:10.1038/nri3152
- Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996; 17(3):138-146. https://doi.org/10.1016/0167-5699(96)80606-2
- Tizard IR. Veterinary Immunology. 10st Edn. Elsevier Health Sciences; 2016.
- Haig D, Deane D, Percival A, Myatt N, Thomson J, Inglis L, et al. The cytokine response of afferent lymph following orf virus reinfection of sheep. Vet Dermatol. 1996; (7)1:11-20. https://doi.org/10.1111/j.1365-3164.1996.tb00221.x
- Anziliero D, Weiblen R, Kreutz LC, Spilki F and Flores, EF. Inactivated Parapoxvirus ovis induces a transient increase in the expression of proinflammatory, Th1-related, and autoregulatory cytokines in mice. Braz J Med Biol Res. 2014; 47(2):110-118. https://doi.org/10.1590/1414-431X20133358
- Avci O, Bulut O, Dik I. Effects of inactive parapoxvirus ovis on cytokine levels in rats. J Vet Med Sci. 2015; 78(1):129-131. https://doi.org/10.1292/jvms.15-0231
- Wang L, Lu B, Zheng H, Zhang K, Liu X. Parapoxvirus orf virus infection induces an increase in interleukin-8, tumour necrosis factor-α, and decorin in goat skin fibroblast cells. J Vet Sci. 2016; 60(3):239-243. https://doi.org/10.1515/jvetres-2016-0036
- Pacheco IL, Abril N, Morales-Prieto N, Bautista MJ, Zafra R, Escamilla A, et al. Th1/Th2 balance in the liver and hepatic lymph nodes of vaccinated and unvaccinated sheep during acute stages of infection with Fasciola hepatica. Vet Parasitol. 2017; 238:61-65. https://doi.org/10.1016/j.vetpar.2017.03.022
- Alhussien MN, Dang AK. Interaction between stress hormones and phagocytic cells and its effect on the health status of dairy cows: A review. Vet World. 2020; 13(9):1837. https://doi.org/10.14202/vetworld.2020.1837-1848
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann N Y Acad Sci. 2014; 1024(1):138-146. https://doi.org/10.1196/annals.1321.010
- Takahashi A, Flanigan ME, McEwen BS, Russo SJ. Aggression, social stress, and the immune system in humans and animal models. Front Behav Neurosci. 2018; 12:56. https://doi.org/10.3389/fnbeh.2018.00056























