Efectos de amitraz más Parapoxvirus ovis sobre EGF, VEGF, IGF-1 e IGF-2 en la demodicosis generalizada canina
Effects of amitraz plus-Parapoxvirus ovis on EGF, VEGF, IGF-1 and IGF-2 in canine generalized demodicosis

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Mostrar biografía de los autores
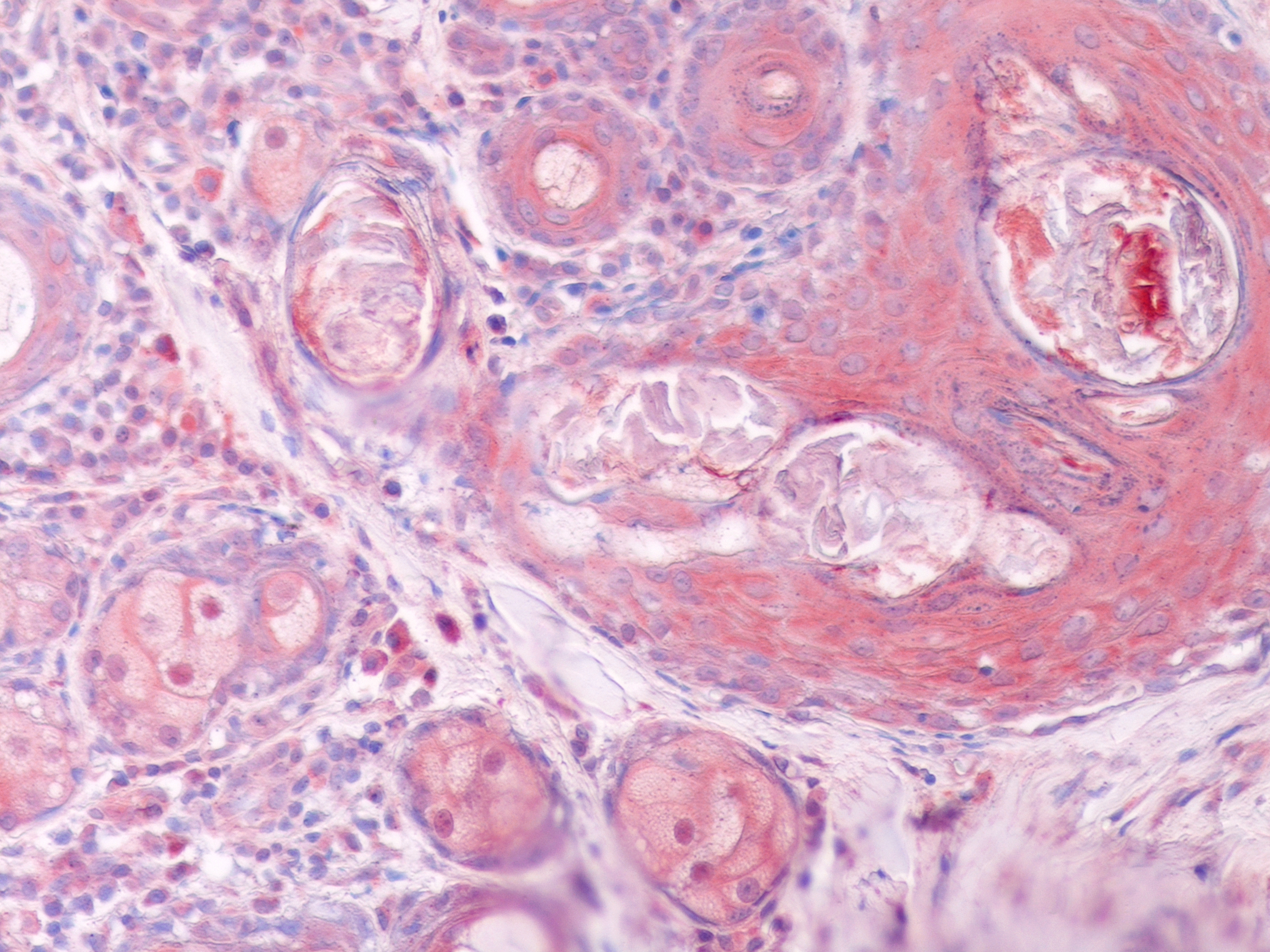
Objetivo. El propósito del estudio es investigar el efecto del tratamiento con amitraz más-Parapoxvirus ovis (IPPVO) sobre las concentraciones séricas y las expresiones cutáneas del factor de crecimiento insulínico (IGF) -1 y -2, factor de crecimiento epidérmico (EGF), vascular factor de crecimiento endotelial (VEGF), en perros que padecen demodicosis generalizada (GD). Materiales y métodos. A los perros afectados por GD se les inyectó 1 mL de IPPVO los días 0, 2 y 9 por vía subcutánea además del tratamiento con amitraz (0,025%) dos veces por semana durante 80 días. Las concentraciones de IGF-1, IGF-2, EGF y VEGF en suero sanguíneo se midieron mediante un kit de ensayo inmunoabsorbente ligado a enzimas específico para caninos. Las expresiones de EGF, VEGF, IGF-1 e IGF-2 en muestras de biopsia de piel se examinaron inmunohistoquímicamente. Resultados. Después del tratamiento de los perros con amitraz más-IPPVO en GD, demostramos una reducción significativa tanto en las concentraciones circulantes como en las expresiones cutáneas de EGF, VEGF, IGF-1 e IGF-2, que tienen un papel en la preservación de la integridad de la piel y la herida. curación. Conclusiones. Los resultados de este estudio sugieren que IGF-1, IGF-2 EGF y VEGF tienen un papel crucial en la progresión de la GD en perros. Se cree que los hallazgos de este estudio contribuirán al desarrollo de nuevas estrategias para el tratamiento de la GD, que es un problema de salud importante para los perros.
Visitas del artículo 358 | Visitas PDF
Descargas
- Gross TL, Ihrke PJ, Walder EJ, Affolter VK. Skin Diseases of the Dog and Cat. Clinical and Histopathologic Diagnosis. Blackwell Publishing: UK; 2005.
- Scott DW, Miller WH, Griffin CE. Parasitic skin diseases. Small animal dermatology. Muller GH and Kirk RW (eds), W.B. Saunders Co: Philadelphia; 2001.
- Day MJ. An immunohistochemical study of the lesions of demodicosis in the dog. J Comp Pathol. 1997; 116:203-216. https://doi.org/10.1016/S0021-9975(97)80077-1
- Shipstone M. Generalised demodicosis in dogs, clinical perspective. Aust Vet J. 2000; 78:240-242. https://doi.org/10.1111/j.1751-0813.2000.tb11741.x
- Tarallo VD, Lia RP, Sasanelli M, Cafarchia C, Otranto D. Efficacy of Amitraz plus Metaflumizone for the treatment of canine demodicosis associated with Malassezia pachydermatis. Parasit Vectors. 2009; 2:13. http://https//doi.org/10.1186/1756-3305-2-13
- Fourie J, Dumont P, Halos L, Beugnet F, Pollmeier M. Efficacy of a topical application of Certifect(R) (fipronil 6.26% w/v, amitraz 7.48% w/v, (S)-methoprene 5.63% w/v) for the treatment of canine generalized demodicosis. Parasite 2013; 20:46. https://doi.org/10.1051/parasite/2013046
- Pekmezci D, Pekmezci GZ, Guzel M, Cenesiz S, Gurler AT, Gokalp G. Efficacy of amitraz plus inactivated Parapoxvirus ovis in the treatment of canine generalised demodicosis. Vet Rec. 2014; 174:556. https://doi.org/10.1136/vr.102226
- Gartner MH, Benson JD, Caldwell MD. Insulin-like growth factors I and II expression in the healing wound. J Surg Res. 1992; 52:389-394. https://doi.org/10.1016/0022-4804(92)90121-F
- Corral CJ, Siddiqui A, Wu L, Farrell CL, Lyons D, Mustoe TA. Vascular endothelial growth factor is more important than basic fibroblastic growth factor during ischemic wound healing. Arch Surg. 1999; 134:200-205. http://dx.doi.org/10.1001/archsurg.134.2.200
- Gibbs S, Silva Pinto AN, Murli S, Huber M, Hohl D, Ponec M. Epidermal growth factor and keratinocyte growth factor differentially regulate epidermal migration, growth, and differentiation. Wound Repair Regen. 2000; 8:192-203. https://doi.org/10.1046/j.1524-475x.2000.00192.x
- Bao P, Kodra A, Tomic-Canic M, Golinko MS, Ehrlich HP, Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009; 153:347-358. https://doi.org/10.1016/j.jss.2008.04.023
- Shirakata Y. Regulation of epidermal keratinocytes by growth factors. J Dermatol Sci. 2010; 59:73-80. https://doi.org/10.1016/j.jdermsci.2010.05.002
- Talebpour Amiri F, Fadaei Fathabadi F, Mahmoudi Rad M, Piryae A, Ghasemi A, Khalilian A, Yeganeh F, Mosaffa N. The effects of insulin-like growth factor-1 gene therapy and cell transplantation on rat acute wound model. Iran Red Crescent Med J. 2014; 16(10):e16323. https://doi.org/10.5812/ircmj.16323
- Wu Z, Tang Y, Fang H, Su Z, Xu B, Lin Y, Zhang P, Wei X. Decellularized scaffolds containing hyaluronic acid and EGF for promoting the recovery of skin wounds. J Mater Sci Mater Med. 2015; 1:5322. https://doi.org/10.1007/s10856-021-06531-9
- Acosta JB, Savigne W, Valdez C, Franco N, Alba JS, del Rio A, López-Saura P, Guillén G, Lopez E, Herrera L, Férnandez-Montequín J. Epidermal growth factor intralesional infiltrations can prevent amputation in patients with advanced diabetic foot wounds. Int Wound J. 2006; 3:232-239. https://doi.org/10.1111/j.1742-481X.2006.00237.x
- Hong JP, Jung HD, Kim YW. Recombinant human epidermal growth factor (EGF) to enhance healing for diabetic foot ulcers. Ann Plast Surg. 2006; 56:394-398. https://doi.org/10.1097/01.sap.0000198731.12407.0c
- Frank S, Hübner G, Breier G, Longaker MT, Greenhalgh DG, Werner S. Regulation of vascular endothelial growth factor expression in cultured keratinocytes. Implications for normal and impaired wound healing. J Biol Chem. 1995; 270:12607-12613. https://doi.org/10.1074/jbc.270.21.12607
- Iijima K, Yoshikawa N, Connolly DT, Nakamura H. Human mesangial cells and peripheral blood mononuclear cells produce vascular permeability factor. Kidney Int. 1993; 44:959-966. https://doi.org/10.1038/ki.1993.337
- Boocock CA, Charnock-Jones DS, Sharkey AM, McLaren J, Barker PJ, Wright KA, Twentyman PR, Smith SK. Expression of vascular endothelial growth factor and its receptors flt and KDR in ovarian carcinoma. J Natl Cancer Inst. 1995; 87:506-516. https://doi.org/10.1093/jnci/87.7.506
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med. 1999; 6:623-628. https://doi.org/10.1038/9467
- Bates DO, Beazley-Long N, Benest AV, Ye X, Ved N, Hulse RP, Barratt S, Machado MJ, Donaldson LF, Harper SJ, Peiris-Pages M, Tortonese DJ, Oltean S, Foster RR. Physiological role of vascular endothelial growth factors as homeostatic regulators. Compr Physiol. 2018; 8:955-979. https://doi.org/10.1002/cphy.c170015
- Zachary I. Molecules in focus VEGF. Int J Biochem Cell Biol. 1998; 30:1169-1174. https://doi.org/10.1016/S1357-2725(98)00082-X
- Yoo H, Kim SJ, Kim Y, Lee H, Kim TY. Insulin-like growth factor-II regulates the 12-lipoxygenase gene expression and promotes cell proliferation in human keratinocytes via the extracellular regulatory kinase and phosphatidylinositol 3-kinase pathways. Int J Biochem Cell Biol. 2007; 39:1248-1259. https://doi.org/10.1016/j.biocel.2007.04.009
- Bohnsack RN, Warejcka DJ, Wang L, Gillespie SR, Bernstein AM, Twining SS, Dahms NM. Expression of insulin-like growth factor 2 receptor in corneal keratocytes during differentiation and in response to wound healing. Invest Ophthalmol Vis Sci. 2014; 55:7697-7708. https://doi.org/10.1167/iovs.14-15179
- Dong ZZ, Yao M, Qian J, Yan XD, Chen J, Yan MJ, Yao NH, Yao DF. Abnormal expression of insulin-like growth factor-II and intervening of its mRNA transcription in the promotion of HepG2 cell apoptosis. Zhonghua Yi Xue Za Zhi. 2013; 93:892-896. https://doi.org/10.3760/cma.j.issn.0376-2491.2013.12.004
- Tani K, Morimoto M, Hayashi T, Inokuma H, Ohnishi T, Hayashiya S, Nomura T, Une S, Nakaichi M, Taura Y. Evaluation of cytokine messenger RNA expression in peripheral blood mononuclear cells from dogs with canine demodicosis. J Vet Med Sci. 2002; 64:513-518. https://doi.org/10.1292/jvms.64.513
- Yarim GF, Yagci BB, Ciftci G. Increased circulating concentrations of PDGF-BB and TGF-β1 in canine generalised demodicosis. Rev Med Vet. 2013; 164:13-17. https://www.researchgate.net/publication/283055446
- Yarim GF, Yagci BB, Yarim M, Sozmen M, Pekmezci D, Cenesiz S, Pekmezci GZ, Karaca E. Serum concentration and skin tissue expression of insulin-like growth factor 2 in canine generalized demodicosis. Vet Dermatol. 2015; 26: 421–e99. https://doi.org/10.1111/vde.12270
- Pereira AV, Pereira SA, Gremião IDF, Campos MP, Ferreira AMR. Comparison of acetate tape impression with squeezing versus skin scraping for the diagnosis of canine demodicosis. Aust Vet J. 2012; 90:448-450. https://doi.org/10.1111/j.1751-0813.2012.00994.x
- Kuznetsova E, Bettenay S, Nikolaeva L, Majzoub M, Mueller R. Influence of systemic antibiotics on the treatment of dogs with generalized demodicosis. Vet Parasitol. 2012; 188:148-155. https://doi.org/10.1016/j.vetpar.2012.02.023
- Lu Z, Weniger M, Jiang K, Boeck S, Zhang K, Bazhin A, Miao Y, Werner J, D'Haese JG. Therapies targeting the tumor stroma and the VEGF/VEGFR axis in pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Target Oncol. 2018; 13:447-459. https://doi.org/10.1007/s11523-018-0578-x
- Wu Q, Wang JH, Li ZQ, Ren JL, Wu YH. Expression of vascular endothelial growth factor in deep second-degree scald wounds in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2014; 36:650-653. https://doi.org/10.3881/j.issn.1000-503X.2014.06.017
- Sobczyńska-Rak A, Polkowska I, Silmanowicz P. Elevated Vascular Endothelial Growth Factor (VEGF) levels in the blood serum of dogs with malignant neoplasms of the oral cavity. Acta Vet Hung. 2014; 62:362-371. https://doi.org/10.1556/avet.2014.009
- Balicki I, Sobczyńska-Rak A. Serum vascular endothelial growth factor concentration in dogs diagnosed with chronic superficial keratitis. Acta Vet Hung. 2014; 62:22-32. https://doi.org/10.1556/avet.2013.040
- Mukorera V, Kirberger RM, Mabeta P, Dvir E. Vascular endothelial growth factor concentrations in dogs with spirocercosis. J Vet Intern Med. 2013; 27:1642-1645. https://doi.org/10.1111/jvim.12179
- Neely EK, Morhenn VB, Hintz RL, Wilson DM, Rosenfeld RG. Insulin-like growth factors are mitogenic for human keratinocytes and a squamous cell carcinoma. J Invest Dermatol. 1991; 96:104-110. https://doi.org/10.1111/1523-1747.ep12515914
- Maeng YS, Choi HJ, Kwon JY, Park YW, Choi KS, Min JK, Kim YH, Suh PG, Kang KS, Won MH, Kim YM, Kwon YG. Endothelial progenitor cell homing: prominent role of the IGF2-IGF2R-PLCbeta2 axis. Blood. 2009; 113:233-243. https://doi.org/10.1182/blood-2008-06-162891























