Efectos de los disruptores endocrinos en la reproducción de rumiantes
Endocrine disruptors effects in ruminant reproduction

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-CompartirIgual 4.0.
Mostrar biografía de los autores
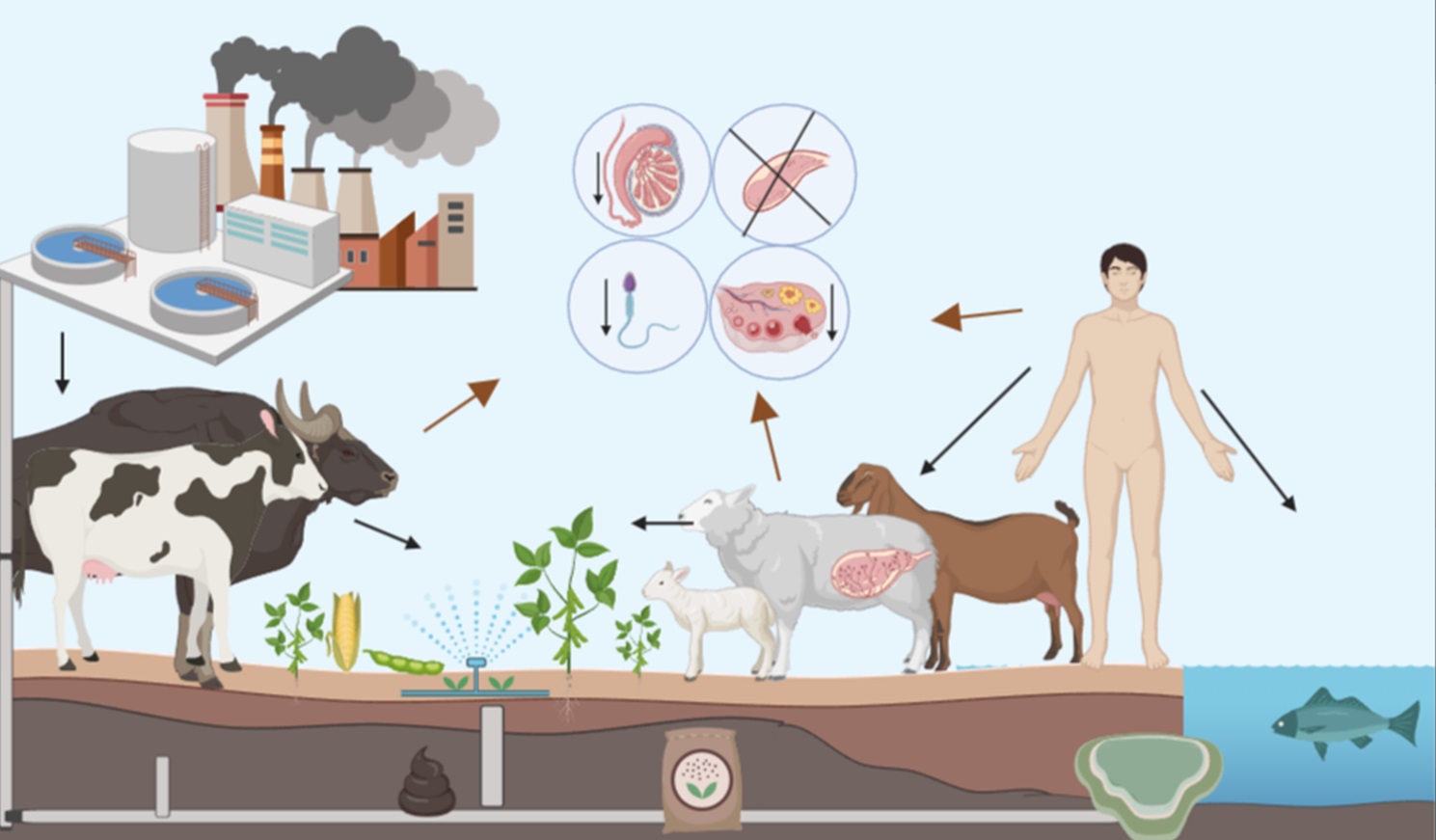
La exposición oral a disruptores endocrinos (DE) sintéticos y naturales en las distintas etapas de la vida está relacionada con alteraciones en el sistema reproductivo. Los rumiantes representan modelos adecuados para estudiar el impacto de los DE en los humanos por algunas similitudes en su desarrollo, se consideran de gran importancia por la exposición continua a pastura y suelo contaminado, y por el consumo humano de su carne. El objetivo de la presente revisión es describir los efectos producidos por consumir DE en etapa perinatal, posnatal-preadultez y adultez en rumiantes. La alimentación con forraje rico en fitoestrógenos (FE), no tiene efectos nocivos sobre la reproducción de machos en ninguna etapa de la vida; en las hembras, producen mayores efectos nocivos cuando se administran en la adultez. En la etapa perinatal y posnatal-preadultez, se encontraron efectos negativos de los DE sintéticos, tanto en machos como en hembras. La presente revisión da a conocer las oportunidades de estudios para continuar con investigaciones relacionadas a la exposición oral a DE.
Visitas del artículo 648 | Visitas PDF
Descargas
- Carson R. Silent spring: 25th anniversary ed. New York: Houghton Mifflin Co.; 1987. http://www.rachelcarson.org/SilentSpring.aspx
- TEDX. The Endocrine Disruption Exchange List of Potential Endocrine Disruptors; 2018. http://www.endocrinedisruption.org/interactive-tools/tedx-list-of-potential-endocrine-disruptors/search-the-tedx-list
- Vandenberg LN, Blumberg B, Antoniou MN, Benbrook CM, Carroll L, Colborn T, et al. Is it time to reassess current safety standards for glyphosate-based herbicides? J Epidemiol Community Health. 2017; 71(6):613-618. http://dx.doi.org/10.1136/jech-2016-208463
- Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009; 30(1):75-95. https://doi.org/10.1210/er.2008-0021
- Colborn T, vom Saal FS, Soto AM. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ Health Perspect. 1993; 101(5):378-384. https://doi.org/10.1289/ehp.93101378
- Kwiatkowski CF, Bolden AL, Liroff RA, Rochester JR, Vandenbergh JG. Twenty-Five Years of Endocrine Disruption Science: Remembering Theo Colborn. Environ Health Perspect. 2016; 124(9):A151-A154. https://doi.org/10.1289/EHP746
- Miyagawa S, Lange A, Hirakawa I, Tohyama S, Ogino Y, Mizutani T, et al. Differing species responsiveness of estrogenic contaminants in fish is conferred by the ligand binding domain of the estrogen receptor. Environ Sci Technol. 2014; 48(9):5254-5263. https://doi.org/10.1021/es5002659
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr Rev. 2015; 36(6):593-602. https://doi.org/10.1210/er.2015-1093
- Krizova L, Dadakova K, Kasparovska J, Kasparovsky T. Isoflavones. Molecules. 2019; 24(6):1076. https://doi.org/10.3390/molecules24061076
- Bhagwat S, Haytowitz DB, Holden JM. USDA Database for the Isoflavone Content of Selected Foods, Release 2.0 Department of Agriculture, Agricultural Research Service; 2008. https://data.nal.usda.gov/dataset/usda-database-isoflavone-content-selected-foods-release-20
- Bennetau-Pelissero C. Risks and benefits of phytoestrogens: where are we now? Curr Opin Clin Nutr Metab Care. 2016; 19(6):477-483. https://doi.org/10.1097/MCO.0000000000000326
- Weber R, Herold C, Hollert H, Kamphues J, Blepp M, Ballschmiter K. Reviewing the relevance of dioxin and PCB sources for food from animal origin and the need for their inventory, control and management. Environ Sci Eur. 2018; 30(1):42. https://doi.org/10.1186/s12302-018-0166-9
- Magnusson U. Environmental endocrine disruptors in farm animal reproduction: research and reality. Reprod Domest Anim. 2012; 47(Suppl 4):333-337. https://doi.org/10.1111/j.1439-0531.2012.02095.x
- Banihashemi B, Droste RL. Sorption-desorption and biosorption of bisphenol A, triclosan, and 17alpha-ethinylestradiol to sewage sludge. Sci Total Environ. 2014; 487:813-821. https://doi.org/10.1016/j.scitotenv.2013.12.116
- Eskenazi B, Warner M, Brambilla P, Signorini S, Ames J, Mocarelli P. The Seveso accident: A look at 40 years of health research and beyond. Environ Int. 2018; 121(Pt1):71-84. https://doi.org/10.1016/j.envint.2018.08.051
- Liu J, Lu G, Xie Z, Zhang Z, Li S, Yan Z. Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci Total Environ. 2015; 511:54-62. https://doi.org/10.1016/j.scitotenv.2014.12.033
- Oskam IC, Lyche JL, Krogenaes A, Thomassen R, Skaare JU, Wiger R, et al. Effects of long-term maternal exposure to low doses of PCB126 and PCB153 on the reproductive system and related hormones of young male goats. Reproduction. 2005; 130(5):731-742. https://doi.org/10.1530/rep.1.00690
- Pietron W, Pajurek M, Mikolajczyk S, Maszewski S, Warenik-Bany M, Piskorska-Pliszczynska J. Exposure to PBDEs associated with farm animal meat consumption. Chemosphere. 2019; 224:58-64. https://doi.org/10.1016/j.chemosphere.2019.02.067
- Yang C, Song G, Lim W. Effects of endocrine disrupting chemicals in pigs. Environ Pollut. 2020; 263(PtB):114505. https://doi.org/10.1016/j.envpol.2020.114505
- Bellingham M, Fowler PA, Amezaga MR, Whitelaw CM, Rhind SM, Cotinot C, et al. Foetal hypothalamic and pituitary expression of gonadotrophin-releasing hormone and galanin systems is disturbed by exposure to sewage sludge chemicals via maternal ingestion. J Neuroendocrinol. 2010; 22(6):527-533. https://doi.org/10.1111/j.1365-2826.2010.01974.x
- Guvvala PR, Ravindra JP, Selvaraju S. Impact of environmental contaminants on reproductive health of male domestic ruminants: a review. Environ Sci Pollut Res Int. 2020; 27(4):3819-3836. https://doi.org/10.1007/s11356-019-06980-4
- Destouni A, Zamani Esteki M, Catteeuw M, Tsuiko O, Dimitriadou E, Smits K, et al. Zygotes segregate entire parental genomes in distinct blastomere lineages causing cleavage-stage chimerism and mixoploidy. Genome Res. 2016; 26(5):567-578. https://doi.org/10.1101/gr.200527.115
- Fishman EL, Jo K, Nguyen QPH, Kong D, Royfman R, Cekic AR, et al. A novel atypical sperm centriole is functional during human fertilization. Nat Commun. 2018; 9(1):2210. https://doi.org/10.1038/s41467-018-04678-8
- Beard AP, Bartlewski PM, Chandolia RK, Honaramooz A, Rawlings NC. Reproductive and endocrine function in rams exposed to the organochlorine pesticides lindane and pentachlorophenol from conception. J Reprod Fertil. 1999; 115(2):303-314. https://doi.org/10.1530/jrf.0.1150303
- Fowler PA, Dora NJ, McFerran H, Amezaga MR, Miller DW, Lea RG, et al. In utero exposure to low doses of environmental pollutants disrupts fetal ovarian development in sheep. Mol Hum Reprod. 2008; 14(5):269-280. https://doi.org/10.1093/molehr/gan020
- Kraugerud M, Aleksandersen M, Nyengaard JR, Ostby GC, Gutleb AC, Dahl E, et al. In utero and lactational exposure to PCB 118 and PCB 153 alter ovarian follicular dynamics and GnRH-induced luteinizing hormone secretion in female lambs. Environ Toxicol. 2012; 27(11):623-634. https://doi.org/10.1002/tox.20679
- Lyche JL, Oskam IC, Skaare JU, Reksen O, Sweeney T, Dahl E, et al. Effects of gestational and lactational exposure to low doses of PCBs 126 and 153 on anterior pituitary and gonadal hormones and on puberty in female goats. Reprod Toxicol. 2004; 19(1):87-95. https://doi.org/10.1016/j.reprotox.2004.05.005
- Senger PL. Pathways to Pregnancy and Parturition. 2nd ed. WA, USA: Current Concepts Inc., Washington State University, Pullman; 2003. https://www.worldcat.org/title/pathways-to-pregnancy-and-parturition/oclc/53250647
- Foster DL. Puberty in the sheep. In: Knobil E, Neil JD, editors. The Physiology of Reproduction. New York: Raven Press, Ltd.; 1994. https://onlinelibrary.wiley.com/doi/abs/10.1002/mrd.1080380414
- Yurrita SC. Evaluation of dietary phytoestrogen exposure on growth, semen parameters, and reproductive anatomy development of growing Angus bulls. U.S.: Angelo State University; 2017. https://asu-ir.tdl.org/bitstream/handle/2346.1/30677/YURRITA-THESIS-2017.pdf?sequence=1&isAllowed=y
- Pace V, Carbone K, Spirito F, Iacurto M, Terzano MG, Verna M, et al. The effects of subterranean clover phytoestrogens on sheep growth, reproduction and carcass characteristics. Meat Sci. 2006; 74(4):616-622. https://doi.org/10.1016/j.meatsci.2006.05.006
- Savoie S, Forest MG, Bourel B, Saez JM, Collu R, Bertrand J, et al. Perinatal activity of the hypothalamic-pituitary-gonadal axis in the lamb. I. Circulating levels of LH, FSH, prolactin and testosterone and in vivo response to hCG in the first two months of life. Biol Reprod. 1979; 21(5):1051-1056. https://doi.org/10.1095/biolreprod21.5.1051
- Amir D, Volcani R. The effect of dietary ethylene dibromide (EDB) on the testes of bulls. A preliminary report. Fertil Steril. 1967; 18:144–147. https://doi.org/10.1016/s0015-0282(16)36196-9
- Woclawek-Potocka I, Acosta TJ, Korzekwa A, Bah MM, Shibaya M, Okuda K, et al. Phytoestrogens modulate prostaglandin production in bovine endometrium: cell type specificity and intracellular mechanisms. Exp Biol Med (Maywood). 2005; 230(5):326-333. https://doi.org/10.1177/153537020523000506.
- Piotrowska KK, Woclawek-Potocka I, Bah MM, Piskula MK, Pilawski W, Bober A, et al. Phytoestrogens and their metabolites inhibit the sensitivity of the bovine corpus luteum to luteotropic factors. J Reprod Dev. 2006; 52(1):33-41. https://doi.org/10.1262/jrd.17054
- García DC, Martín AA, Vella MA, Nasca JA, Roldan EM. Evaluación de la alimentación con distintos niveles de inclusión de soja en la recría de vaquillonas. Veter Arg. 2018; 35(357):1-9. https://www.veterinariargentina.com/revista/2018/11/evaluacion-de-la-alimentacion-con-distintos-niveles-de-inclusion-de-soja-en-la-recria-de-vaquillonas/
- Hashem NM, El-Azrak KM, Sallam SM. Hormonal concentrations and reproductive performance of Holstein heifers fed Trifolium alexandrinum as a phytoestrogenic roughage. Anim Reprod Sci. 2016; 170:121-127. https://doi.org/10.1016/j.anireprosci.2016.04.012
- Pace V, Contò G, Carfì F, Chiariotti A, Catillo G. Short- and long-term effects of low estrogenic subterranean clover on ewe reproductive performance. Small Rumin Res. 2011; 97(1-3):94-100. https://doi.org/10.1016/j.smallrumres.2011.02.011
- Sierra LA. Evaluación del efecto de los fitoestrógenos presentes en la alfalfa (Medicago sativa) sobre la calidad del semen ovino fresco y criopreservado. Colombia, Tunja: Univesidad Pedagógica y Tecnológica de Colombia, Tunja; 2015. https://repositorio.uptc.edu.co/handle/001/2260
- Aragadvay-Yungán R, Novillo-Rueda M, Núñez-Torres O, Rosero-Peña M, Lozada-Salcedo E. Calidad seminal de carneros alimentados con dietas que contienen alfalfa (medicago sativa) contaminada con pseudopeziza medicaginis. Rev Ecuatoriana In Agropecu. 2018; 2(1): 14-19. http://dx.doi.org/10.31164/reiagro.v2n1.3
- Rodríguez MCE. Determinación del contenido de fitoestrógenos presentes en la alfalfa (medicago sativa) y el trébol rojo (trifolium pratense) y evaluación de su efecto sobre el perfil hormonal en vacas Holstein en la Granja Tungüavita Paipa-Boyacá. Colombia: Universidad Pedagógica y Tecnológica de Colombia; 2013. https://repositorio.uptc.edu.co/handle/001/2262
- Hashem NM, El-Azrak KM, Nour El-Din ANM, Sallam SM, Taha TA, Salem MH. Effects of Trifolium alexandrinum phytoestrogens on oestrous behaviour, ovarian activity and reproductive performance of ewes during the non-breeding season. Anim Reprod Sci. 2018; 196:1-8. https://doi.org/10.1016/j.anireprosci.2018.03.007
- Cantero A, Sancha JL, Flores JM, Rodriguez A, Gonzalez T. Histopathological changes in the reproductive organs of Manchego ewes grazing on lucerne. Zentralbl Veterinarmed A. 1996; 43(6):325-330. https://doi.org/10.1111/j.1439-0442.1996.tb00459.x
- Kiyama R, Wada-Kiyama Y. Estrogenic endocrine disruptors: Molecular mechanisms of action. Environ Int. 2015; 83:11-40. https://doi.org/10.1016/j.envint.2015.05.012
- Brouwer A, Longnecker MP, Birnbaum LS, Cogliano J, Kostyniak P, Moore J, et al. Characterization of potential endocrine-related health effects at low-dose levels of exposure to PCBs. Environ Health Perspect. 1999; 107(Suppl 4):639-649. https://doi.org/10.1289/ehp.97105294
- Fang H, Tong W, Branham WS, Moland CL, Dial SL, Hong H, et al. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem Res Toxicol. 2003; 16(10):1338-1358. https://doi.org/10.1021/tx030011g
- Jung J, Ishida K, Nishikawa J, Nishihara T. Inhibition of estrogen action by 2-phenylchromone as AhR agonist in MCF-7 cells. Life Sci. 2007; 81(19-20):1446-1451. https://doi.org/10.1016/j.lfs.2007.09.010
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, et al. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998; 139(10):4252-4263. https://doi.org/10.1210/endo.139.10.6216
- UN. Shaping our future together. Shifting Demographics: United Nations; 2020. https://www.un.org/en/un75/shifting-demographics
- Ritchie H, Max R. Meat and Dairy Production England: Our World in Data; 2019. https://ourworldindata.org/meat-production
- FAO. Human energy requirements, Report of a Joint FAO/WHO/UNU Expert Consultation Rome: United Nations University, World Health Organization, Food and Agriculture Organization of the United Nations; 2001. http://www.fao.org/3/y5686e/y5686e00.htm.
- Kamarianos A, Karamanlis X, Theodosiadou E, Goulas P, Smokovitis A. The presence of environmental pollutants in the semen of farm animals (bull, ram, goat, and boar). Reprod Toxicol. 2003; 17(4):439-445. https://doi.org/10.1016/s0890-6238(03)00031-5
- Xie Z, Lu G, Liu J, Yan Z, Ma B, Zhang Z, et al. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere. 2015; 138:140-147. https://doi.org/10.1016/j.chemosphere.2015.05.086
- de Oliveira M, Frihling BEF, Velasques J, Filho F, Cavalheri PS, Migliolo L. Pharmaceuticals residues and xenobiotics contaminants: Occurrence, analytical techniques and sustainable alternatives for wastewater treatment. Sci Total Environ. 2020; 705:135568. https://doi.org/10.1016/j.scitotenv.2019.135568
- Cao T, Cao Y, Wang H, Wang P, Wang X, Niu H, et al. The Effect of Exposure to Bisphenol A on Spermatozoon and the Expression of Tight Junction Protein Occludin in Male Mice. Dose Response. 2020; 18(2):1559325820926745. https://doi.org/10.1177/1559325820926745























