Cultivo de células epiteliales dentales: impacto del suero fetal bovino
Culture of dental epithelial cells: impact of fetal bovine serum
Mostrar biografía de los autores
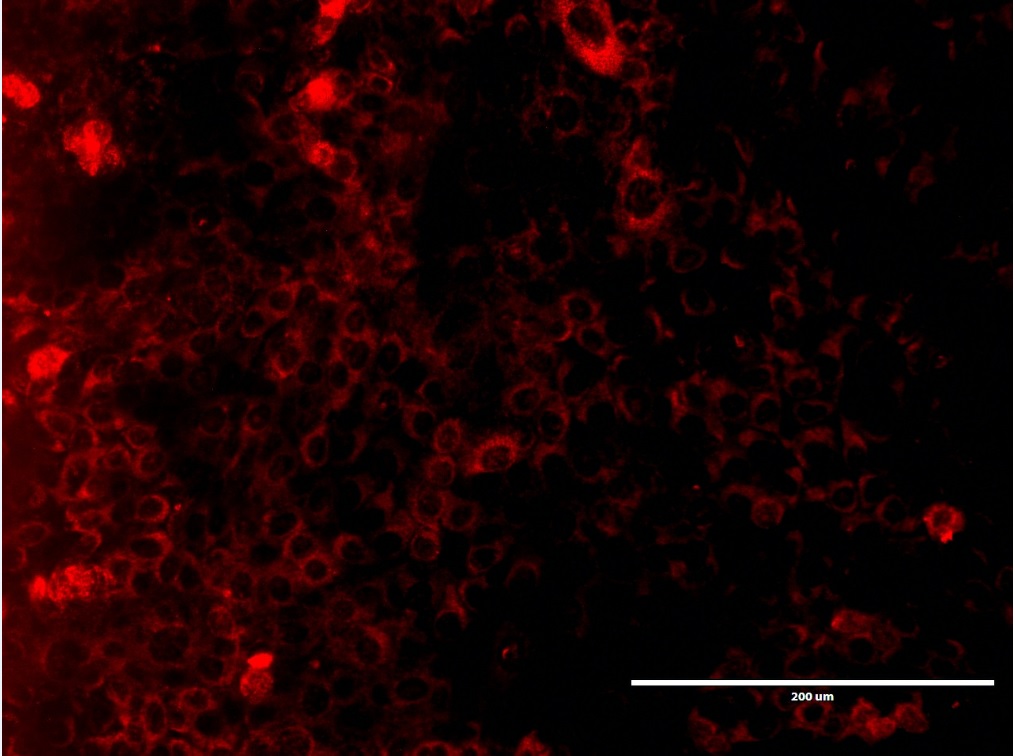
Objetivo. Describir la influencia del Suero Fetal Bovino (SFB) en la supervivencia, crecimiento y expresión de organelas celulares en las células epiteliales dentales de rata. Materiales y métodos. Cultivos de células epiteliales dentales de rata fueron llevados a cabo a 37°C en una atmosfera húmeda, en ausencia y a una concentración de 10% de SFB. Una evaluación morfológica fue realizada durante la proliferación y confluencia de las células en cultivo. Dobles marcajes por inmunofluorencia fueron efectuados haciendo uso de anticuerpos anti-actina, anti-TOMM20 y anti-LAMP1. Resultados. Se evidenciaron células epiteliales dentales circulares u ovoides con núcleos voluminosos durante la proliferación y confluencias de manera similar en las células cultivas en presencia y ausencia de SFB. La carencia de SFB impactó negativamente la proliferación de las células epiteliales. No fueron observadas alteraciones en la localización de los inmunomarcajes anti-actina, anti-TOMM20 y anti-LAMP1 en las dos condiciones de cultivos experimentales. Conclusiones. La supresión del SFB en el cultivo de células epiteliales dentales de rata disminuyó la supervivencia, proliferación y sugiere no tener un impacto sobre las organelas evaluadas.
Visitas del artículo 1486 | Visitas PDF
Descargas
- Yuan Y, Chai Y. Chapter Four - Regulatory mechanisms of jaw bone and tooth development. In: Olsen BR, editor. Vertebrate Skeletal Development. Academic Press; 2019. 133:91–118. https://doi.org/10.1016/bs.ctdb.2018.12.013
- Balic A, Thesleff I. Chapter Seven - Tissue Interactions Regulating Tooth Development and Renewal. Curr Top Dev Biol. 2015; 115:157-186. https://doi.org/10.1016/bs.ctdb.2015.07.006
- Moradian-Oldak J. Protein-mediated enamel mineralization. Front Biosci (Landmark Ed). 2012; 17:1996–2023. http://dx.doi.org/10.2741/4034
- Lacruz RS. Enamel: Molecular identity of its transepithelial ion transport system. Cell Calcium. 2017; 65:1–7. https://doi.org/10.1016/j.ceca.2017.03.006
- Warshawsky H, Josephsen K, Thylstrup A, Fejerskov O. The development of enamel structure in rat incisors as compared to the teeth of monkey and man. The Anatomical Record. 1981; 200(4):371–399. https://doi.org/10.1002/ar.1092000402
- Thesleff I. From understanding tooth development to bioengineering of teeth. European Journal of Oral Sciences. 2018; 126(S1):67–71. https://doi-org.gate2.inist.fr/10.1111/eos.12421
- Kawano S, Morotomi T, Toyono T, Nakamura N, Uchida T, Ohishi M, et al. Establishment of dental epithelial cell line (HAT-7) and the cell differentiation dependent on Notch signaling pathway. Connect Tissue Res. 2002; 43(2–3):409–412. https://doi.org/10.1080/03008200290000637
- Kawasaki K. The SCPP Gene Family and the Complexity of Hard Tissues in Vertebrates. Cells Tissues Organs. 2011; 194(2–4):108–112. https://doi-org.gate2.inist.fr/10.1159/000324225
- Nakamura T, Chiba Y, Naruse M, Saito K, Harada H, Fukumoto S. Globoside accelerates the differentiation of dental epithelial cells into ameloblasts. International Journal Of Oral Science. 2016; 8:205. https://doi.org/10.1038/ijos.2016.35
- Matsumoto A, Harada H, Saito M, Taniguchi A. Induction of enamel matrix protein expression in an ameloblast cell line co-cultured with a mesenchymal cell line in vitro. In Vitro Cell Dev Biol Anim. 2011; 47(1):39–44. https://doi.org/10.1007/s11626-010-9362-7
- Varga G, DenBesten P, Rácz R, Zsembery Á. Importance of bicarbonate transport in pH control during amelogenesis - need for functional studies. Oral Dis. 2018; 24(6):879–890. https://doi.org/10.1111/odi.12738
- Abdel Moniem E, Mahmoud EL-Batran M, Mahmoud Halawa A, Hazem Gomaa D, Nour Eldeen G, Mohamed Aly R. Optimizing a serum-free/xeno-free culture medium for culturing and promoting the proliferation of human dental pulp stem cells. Stem Cell Investig 2019; 6:15. http://dx.doi.org/10.21037/sci.2019.06.05
- Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016; 76:371–387. https://doi.org/10.1016/j.biomaterials.2015.10.065
- Valk J van der, Bieback K, Buta C, Cochrane B, Dirks WG, Fu J, et al. Fetal bovine serum (FBS): Past – present – future. 1. ALTEX. 2018; 35(1):99–118. https://doi.org/10.14573/altex.1705101
- Rácz R, Földes A, Bori E, Zsembery Á, Harada H, Steward MC, et al. No Change in Bicarbonate Transport but Tight-Junction Formation Is Delayed by Fluoride in a Novel Ameloblast Model. Front Physiol. 2017; 8:940. https://doi.org/10.3389/fphys.2017.00940
- Park S-J, Lee H-K, Seo Y-M, Son C, Bae HS, Park J-C. Dentin sialophosphoprotein expression in enamel is regulated by Copine-7, a preameloblast-derived factor. Archives of Oral Biology. 2018; 86:131–137. https://doi.org/10.1016/j.archoralbio.2017.12.004
- Baker M. Reproducibility: Respect your cells! Nature. 2016; 537:433–435. https://doi.org/10.1038/537433a
- Wei Z, Batagov AO, Carter DRF, Krichevsky AM. Fetal Bovine Serum RNA Interferes with the Cell Culture derived Extracellular RNA. Sci Rep. 2016; 6:31175. https://doi.org/10.1038/srep31175
- van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol In Vitro. 2004; 18(1):1–12. https://doi.org/10.1016/j.tiv.2003.08.009
- Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016; 76:371–387. https://doi.org/10.1016/j.biomaterials.2015.10.065
- Wu M-F, Stachon T, Seitz B, Langenbucher A, Szentmáry N. Effect of human autologous serum and fetal bovine serum on human corneal epithelial cell viability, migration and proliferation in vitro. Int J Ophthalmol. 2017; 10(6):908–913. https://doi.org/10.18240/ijo.2017.06.12
- Yue B. Biology of the Extracellular Matrix: An Overview. J Glaucoma. 2014; S20–3. https://doi.org/10.1097/IJG.0000000000000108
- Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell. 2013; 24(5):447–458. https://doi.org/10.1016/j.devcel.2013.02.012
- Pollard TD. Actin and Actin-Binding Proteins. Cold Spring Harb Perspect Biol. 2016; 8(8):a018226. https://doi.org/10.1101/cshperspect.a018226
- Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010; 27(1):53–62. https://doi.org/10.14573/altex.2010.1.53
- Ritchie C. Protease Inhibitors. Mater Methods. 2013; 3:169. https://doi.org/10.13070/mm.en.3.169
- Arora M. Cell Culture Media: A Review. Mater Methods 2013; 3:175. https://doi.org/10.13070/mm.en.3.175
- Richter U, Lahtinen T, Marttinen P, Myöhänen M, Greco D, Cannino G, et al. A Mitochondrial Ribosomal and RNA Decay Pathway Blocks Cell Proliferation. Current Biology. 2013; 23(6):535–541. https://doi.org/10.1016/j.cub.2013.02.019