Culture of dental epithelial cells: impact of fetal bovine serum
Cultivo de células epiteliales dentales: impacto del suero fetal bovino
Show authors biography
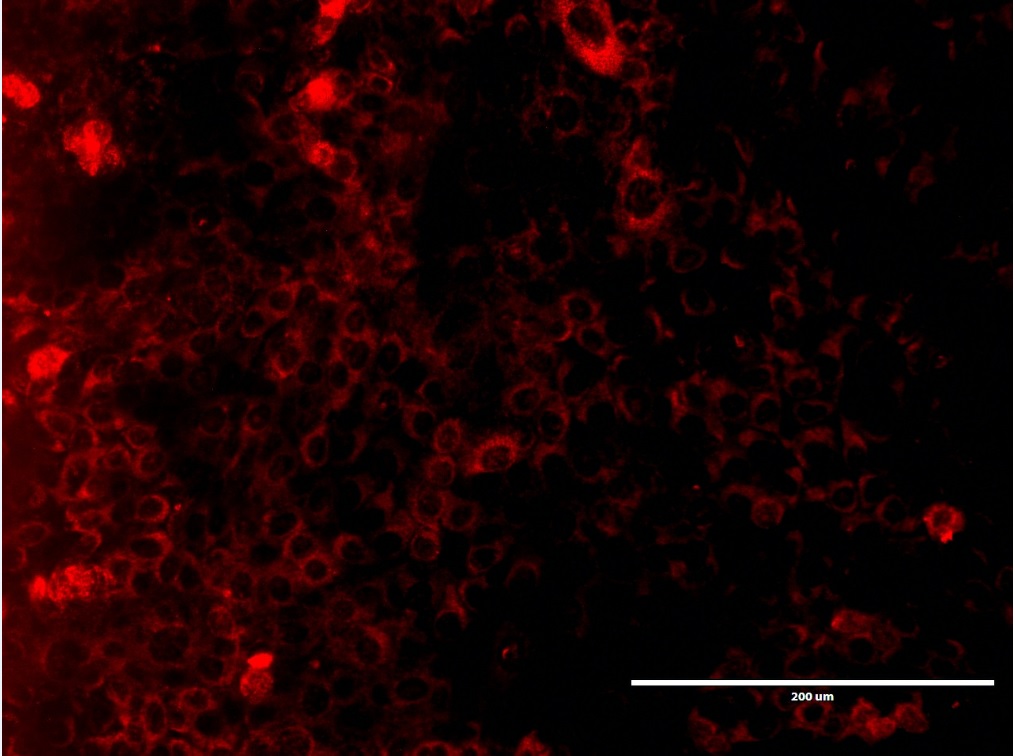
Objective. Describe the influence of Fetal bovine serum (FBS) on the survival, growth and expression of cellular organelles in rat dental epithelial cells. Material and methods. Cell cultures of rat dental epithelial cells were carried out at 37°C in a humid atmosphere, in the absence and at a concentration of 10% FBS. Morphological evaluation was performed during the proliferation and confluence of cell in culture. Double immunofluorescence labels were made using anti-Actin, anti-TOMM20A, and anti-LAMP1 antibodies. Results. Circular or ovoid dental epithelial cells with bulky nuclei were evidenced during proliferation and confluences in a similar manner in culturing cells in the presence and absence of FBS. The lack of FBS negatively impacts the proliferation of epithelial cells. No alterations were observed in the localization of the anti-actin, anti-TOMM20 and anti-LAMP1 immunomarkers in both conditions of experimental cultures. Conclusions. FBS suppression in rat dental epithelial cells decreased survival, proliferation and suggests not having an impact on the organelles evaluated.
Article visits 1485 | PDF visits
Downloads
- Yuan Y, Chai Y. Chapter Four - Regulatory mechanisms of jaw bone and tooth development. In: Olsen BR, editor. Vertebrate Skeletal Development. Academic Press; 2019. 133:91–118. https://doi.org/10.1016/bs.ctdb.2018.12.013
- Balic A, Thesleff I. Chapter Seven - Tissue Interactions Regulating Tooth Development and Renewal. Curr Top Dev Biol. 2015; 115:157-186. https://doi.org/10.1016/bs.ctdb.2015.07.006
- Moradian-Oldak J. Protein-mediated enamel mineralization. Front Biosci (Landmark Ed). 2012; 17:1996–2023. http://dx.doi.org/10.2741/4034
- Lacruz RS. Enamel: Molecular identity of its transepithelial ion transport system. Cell Calcium. 2017; 65:1–7. https://doi.org/10.1016/j.ceca.2017.03.006
- Warshawsky H, Josephsen K, Thylstrup A, Fejerskov O. The development of enamel structure in rat incisors as compared to the teeth of monkey and man. The Anatomical Record. 1981; 200(4):371–399. https://doi.org/10.1002/ar.1092000402
- Thesleff I. From understanding tooth development to bioengineering of teeth. European Journal of Oral Sciences. 2018; 126(S1):67–71. https://doi-org.gate2.inist.fr/10.1111/eos.12421
- Kawano S, Morotomi T, Toyono T, Nakamura N, Uchida T, Ohishi M, et al. Establishment of dental epithelial cell line (HAT-7) and the cell differentiation dependent on Notch signaling pathway. Connect Tissue Res. 2002; 43(2–3):409–412. https://doi.org/10.1080/03008200290000637
- Kawasaki K. The SCPP Gene Family and the Complexity of Hard Tissues in Vertebrates. Cells Tissues Organs. 2011; 194(2–4):108–112. https://doi-org.gate2.inist.fr/10.1159/000324225
- Nakamura T, Chiba Y, Naruse M, Saito K, Harada H, Fukumoto S. Globoside accelerates the differentiation of dental epithelial cells into ameloblasts. International Journal Of Oral Science. 2016; 8:205. https://doi.org/10.1038/ijos.2016.35
- Matsumoto A, Harada H, Saito M, Taniguchi A. Induction of enamel matrix protein expression in an ameloblast cell line co-cultured with a mesenchymal cell line in vitro. In Vitro Cell Dev Biol Anim. 2011; 47(1):39–44. https://doi.org/10.1007/s11626-010-9362-7
- Varga G, DenBesten P, Rácz R, Zsembery Á. Importance of bicarbonate transport in pH control during amelogenesis - need for functional studies. Oral Dis. 2018; 24(6):879–890. https://doi.org/10.1111/odi.12738
- Abdel Moniem E, Mahmoud EL-Batran M, Mahmoud Halawa A, Hazem Gomaa D, Nour Eldeen G, Mohamed Aly R. Optimizing a serum-free/xeno-free culture medium for culturing and promoting the proliferation of human dental pulp stem cells. Stem Cell Investig 2019; 6:15. http://dx.doi.org/10.21037/sci.2019.06.05
- Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016; 76:371–387. https://doi.org/10.1016/j.biomaterials.2015.10.065
- Valk J van der, Bieback K, Buta C, Cochrane B, Dirks WG, Fu J, et al. Fetal bovine serum (FBS): Past – present – future. 1. ALTEX. 2018; 35(1):99–118. https://doi.org/10.14573/altex.1705101
- Rácz R, Földes A, Bori E, Zsembery Á, Harada H, Steward MC, et al. No Change in Bicarbonate Transport but Tight-Junction Formation Is Delayed by Fluoride in a Novel Ameloblast Model. Front Physiol. 2017; 8:940. https://doi.org/10.3389/fphys.2017.00940
- Park S-J, Lee H-K, Seo Y-M, Son C, Bae HS, Park J-C. Dentin sialophosphoprotein expression in enamel is regulated by Copine-7, a preameloblast-derived factor. Archives of Oral Biology. 2018; 86:131–137. https://doi.org/10.1016/j.archoralbio.2017.12.004
- Baker M. Reproducibility: Respect your cells! Nature. 2016; 537:433–435. https://doi.org/10.1038/537433a
- Wei Z, Batagov AO, Carter DRF, Krichevsky AM. Fetal Bovine Serum RNA Interferes with the Cell Culture derived Extracellular RNA. Sci Rep. 2016; 6:31175. https://doi.org/10.1038/srep31175
- van der Valk J, Mellor D, Brands R, Fischer R, Gruber F, Gstraunthaler G, et al. The humane collection of fetal bovine serum and possibilities for serum-free cell and tissue culture. Toxicol In Vitro. 2004; 18(1):1–12. https://doi.org/10.1016/j.tiv.2003.08.009
- Burnouf T, Strunk D, Koh MBC, Schallmoser K. Human platelet lysate: Replacing fetal bovine serum as a gold standard for human cell propagation? Biomaterials. 2016; 76:371–387. https://doi.org/10.1016/j.biomaterials.2015.10.065
- Wu M-F, Stachon T, Seitz B, Langenbucher A, Szentmáry N. Effect of human autologous serum and fetal bovine serum on human corneal epithelial cell viability, migration and proliferation in vitro. Int J Ophthalmol. 2017; 10(6):908–913. https://doi.org/10.18240/ijo.2017.06.12
- Yue B. Biology of the Extracellular Matrix: An Overview. J Glaucoma. 2014; S20–3. https://doi.org/10.1097/IJG.0000000000000108
- Wolfenson H, Lavelin I, Geiger B. Dynamic regulation of the structure and functions of integrin adhesions. Dev Cell. 2013; 24(5):447–458. https://doi.org/10.1016/j.devcel.2013.02.012
- Pollard TD. Actin and Actin-Binding Proteins. Cold Spring Harb Perspect Biol. 2016; 8(8):a018226. https://doi.org/10.1101/cshperspect.a018226
- Brunner D, Frank J, Appl H, Schöffl H, Pfaller W, Gstraunthaler G. Serum-free cell culture: the serum-free media interactive online database. ALTEX. 2010; 27(1):53–62. https://doi.org/10.14573/altex.2010.1.53
- Ritchie C. Protease Inhibitors. Mater Methods. 2013; 3:169. https://doi.org/10.13070/mm.en.3.169
- Arora M. Cell Culture Media: A Review. Mater Methods 2013; 3:175. https://doi.org/10.13070/mm.en.3.175
- Richter U, Lahtinen T, Marttinen P, Myöhänen M, Greco D, Cannino G, et al. A Mitochondrial Ribosomal and RNA Decay Pathway Blocks Cell Proliferation. Current Biology. 2013; 23(6):535–541. https://doi.org/10.1016/j.cub.2013.02.019