Evaluación del efecto acaricida de Momordica charantia, Megaskepasma erythrochlamys y Gliricidia sepium sobre Rhipicephalus microplus
Evaluation of the acaricidal effect of Momordica charantia, Megaskepasma erythrochlamys and Gliricidia sepium on the Rhipicephalus microplus Acaricidal effect of forage plants
Mostrar biografía de los autores
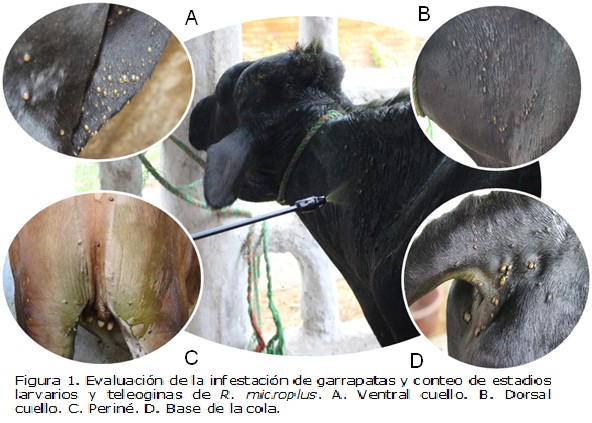
Objetivo. Se evaluó la actividad acaricida de Momordica charantia (Mc), Megaskepasma erythrochlamys (Me) y Gliricidia sepium (Gs) sobre Rhipicephalus microplus (Rm). Materiales y métodos. Se realizó la marcha fitoquímica preliminar de hojas del extracto metanólico de Mc (EMc), del extracto etanólico de Me (EMe) y del extracto acetónico de Gs (EGs) a través de la técnica de colorimetría y cromatografía en capa delgada (CCD). La actividad acaricida se realizó a través de pruebas in-vitro utilizando la prueba de inmersión de larvas (LIT) y la prueba de inmersión de adultos (AIT). Para las pruebas in-situ se usaron bovinos en pastoreo infestados naturalmente con garrapatas, utilizando las CL50 obtenidas en las pruebas in-vitro AIT; posteriormente las teleoginas se llevaron a incubación para evaluar su capacidad reproductiva. Resultados. Se determinó la presencia de varios grupos de metabolitos secundarios de interés acaricida. Se demostró el efecto acaricida de los extractos de las plantas sobre teleoginas; aunque sólo EGs mostró actividad larvicida. Los extractos a 160 mg/mL afectaron el ciclo de vida de Rm inhibiendo la ovoposición en un 46.9%, 66.1% y 84.03% (p<0.05) para EGs, EMc y EMe, respectivamente. Por otro lado, en las pruebas in situ se observó diferencia significativa (p<0.05) entre el tratamiento de EMc y EMe respecto a los grupos controles. Conclusiones. Los resultados obtenidos son prometedores para fortalecer la posibilidad de vinculación de los extractos de estas plantas dentro de planes integrados de control de garrapatas en sistemas de producción de bovinos.
Visitas del artículo 1678 | Visitas PDF
Descargas
- CONPES. Documento CONPES 3676: Consolidación de la política sanitaria y de inocuidad para las cadenas láctea y cárnica. Colombia, Bogotá: Departamento Nacional de Planeación, Consejo Nacional de Política Económica y Social, Departamento Nacional de Planeación. 2010. URL Available from: https://colaboracion.dnp.gov.co/CDT/Conpes/Econ%C3%B3micos/3676.pdf
- [Google Scholar]
- Reck J, Berger M, Terra RM, Marks FS, da Silva I, Guimarães JA, Termignoni C. Systemic alterations of bovine hemostasis due to Rhipicephalus (Boophilus) microplus infestation. Res Vet Sci. 2009; 86(1):56–62. https://doi.org/10.1016/j.rvsc.2008.05.007 [CrossRef] [PubMed] [Google Scholar]
- Bianchi MV, Barré N, Messad S. Factors related to cattle infestation level and resistance to acaricides in Boophilus microplus tick populations in New Caledonia. Vet Parasitol 2003; 112(1-2):75-89. https://doi.org/10.1016/s0304-4017(02)00415-6
- [CrossRef] [PubMed] [Google Scholar]
- FAO. Maximum residue limits (MRLs) and risk management recommendations (RMRs) for residues of veterinary drugs in foods. FAO/WHO Food Standards Programme. Food and Agriculture Organization of the United Nations. 2017. URL Available from: http://www.fao.org/fao-who-codexalimentarius/codex-texts/maximum-residue-limits/en/
- Montes-Molina JA, Luna-Guidoa ML, Espinoza-Paz N, Govaertsc B, Gutierrez-Micelid FA, Dendooven L. Are extracts of neem (Azadirachta indica A. Juss. (L.)) and Gliricidia sepium (Jacquin) an alternative to control pests on maize (Zea mays L.)? Crop Prot 2008; 27(3-5):763–774. https://doi.org/10.1016/j.cropro.2007.11.002
- [CrossRef] [Google Scholar]
- Castelblanco L, Sanabria OJ, Cruz A, Rodríguez CE. Reporte preliminar del efecto ixodicida de extractos de algunas plantas sobre garrapatas Boophilus microplus. Rev Cubana Plant Med 2013; 18(1):118-130. [Google Scholar]
- García-Barriga H. Flora Medicinal de Colombia. Tomos I y II. Bogotá, Colombia: Imprenta Nacional de Colombia; 1975. [Google Scholar]
- Sanabria A. Análisis fitoquímico preliminar. Metodología y su aplicación en la evaluación de 40 plantas de la familia Compositae. Bogotá, Colombia: Universidad Nacional de Colombia, Facultad de Ciencias, Departamento de Farmacia. 1983. https://books.google.com.co/books/about/Analisis_fitoquimico_preliminar_metodolo.html?id=i2vhMgEACAAJ&redir_esc=y
- Drummond RO, Glandey WJ, Whetstone T, Ernest SE. Laboratory testing of insecticides for control of the winter tick. J Econ Entomol 1971; 64(3):686-688. https://doi.org/10.1093/jee/64.3.686 [CrossRef] [PubMed] [Google Scholar]
- Klafke GM, Castro-Janer E, Mendes MC, Namindome A, Schumaker TT. Applicability of in vitro bioassays for the diagnosis of ivermectin resistance in Rhipicephalus microplus (Acari: Ixodidae). Vet Parasitol 2012; 184(2-4):212–220.
- https://doi.org/10.1016/j.vetpar.2011.09.018 [CrossRef] [PubMed] [Google Scholar]
- FAO. Animal Production and Health Division. Resistance Management and Integrated Parasite Control in Ruminants. Italy, Rome: Animal Production and Health Division, Agriculture Dept., Food and Agriculture Organization of the United Nations. 2004. URL Available from: http://www.fao.org/tempref/docrep/fao/010/ag014e/ag014e00.pdf
- Dvorkin-Camiel L, Pharm DJS, Whelan MS. Tropical American Plants in the Treatment of Infectious Diseases. J Diet Suppl 2008; 5(4):349-372. https://doi.org/10.1080/19390210802519648 [CrossRef] [PubMed] [Google Scholar]
- Cuervo JA, Narváez SW, Hahn C. Características forrajeras de la especie Gliricidia sepium (Jacq.) stend, fabaceae. Bol Cient Mus Hist Nat 2013; 17(1):33-45. http://boletincientifico.ucaldas.edu.co/downloads/Boletin17(1)_COMPLETO.pdf
- [Google Scholar]
- Sivira A, Sanabria ME, Valera N, Vásquez C. Toxicity of ethanolic extracts from Lippia origanoides and Gliricidia sepium to Tetranychus cinnabarinus (Boisduval) (Acari: Tetranychidae). Neotrop Entomol 2011; 40(3):375-379. http://dx.doi.org/10.1590/S1519-566X2011000300011 [CrossRef] [PubMed] [Google Scholar]
- Álvarez V, Loaiza J, Bonilla R, Barrios M. Control in vitro tick (Boophilus microplus; Acari: Ixodidae) through plant extracts. Rev Biol Trop 2008; 56(1):291-302. https://doi.org/10.15517/rbt.v56i1.5525 [CrossRef] [PubMed] [Google Scholar]
- Bufford J, Lurie M, Daehler C. Biotic resistance to tropical ornamental invasion. Journal of ecology. 2016, 104:518-530. https://doi.org/10.1111/1365-2745.12534 [Google Scholar]
- Joji Rl, Beena, J. Chemical composition and antibacterial activity of the volatile oil from the bark of Gliricidia sepium. Int J Pharmacy Pharm Sci 2010; 2(3):177-179. https://innovareacademics.in/journal/ijpps/Vol2Issue3/651.pdf
- Krishnappa K, Dhanasekaran S, Elumalai K. Larvicidal, ovicidal and pupicidal activities of Gliricidia sepium (Jacq.) (Leguminosae) against the malarial vector, Anopheles stephensi Liston (Culicidae: Diptera). Asian Pac J Trop Med 2012; 5(8):598-604. https://doi.org/10.1016/S1995-7645(12)60124-2 [CrossRef] [PubMed] [Google Scholar]
- Thomas, J., Govindan, S., Kurup, M. (2014). Isolation and characterization of mosquito larvicidal compound from Gliricidia sepium Jacq. Int J Pharm Res Health Sci 2(2):173-178. http://www.pharmahealthsciences.net/pdfs/volume2/13_MS_1443.pdf
- [Google Scholar]
- von Son-de Fernex E, Alonso-Dayz MA, Valles-de la Mora B, Capetillo-Leal CM. In vitro anthelmintic activity of five tropical legumes on the exsheathment and motility of Haemonchus contortus infective larvae. Exp Parasitol 2012; 131(4):413-8. https://doi.org/10.1016/j.exppara.2012.05.010 [CrossRef] [PubMed] [Google Scholar]
- Puerto M, Arece J, López Y, Roche Y, Molina M, Sanavria A, da Fonseca HA. Efecto in vitro de extracts acuosos de Moringa oleifera y Gliricida sepium en el desarrollo de las fases exógenas de estrongílidos gastrointestinales de ovinos. Rev Salud Anim 2014; 36(1):28-34. http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0253-570X2014000100005
- Bagavan A, Kamara JC, Elango G, Zahir AA, Rahuman AA. Adulticidal and larvicidal efficacy of some medicinal plant extracts against tick, fluke and mosquitoes. Vet Parasitol 2009; 166(3-4):286-292. https://doi.org/10.1016/j.vetpar.2009.09.007
- [CrossRef] [PubMed] [Google Scholar]
- Zorloni A, Penzhorn BL, Eloff JN. Extracts of Calpurnia aurea leaves from southern Ethiopia attract and immobilise or kill ticks. Vet Parasitol 2010; 168(1-2):160-164.
- https://doi.org/10.1016/j.vetpar.2009.10.026 [CrossRef] [PubMed] [Google Scholar]
- Dantas ACS, Machado DMR, Araujo AC, Oliveira-Junior RG, Lima-Saraiva SRG, Ribeiro LAA, Almeida JRGS, Horta MC. Acaricidal activity of extracts from the leaves and aerial parts of Neoglaziovia variegata (Bromeliaceae) on the cattle tick Rhipicephalus (Boophilus) microplus. Res Vet Sci 2015; 100:165–168. https://doi.org/10.1016/j.rvsc.2015.04.012 [CrossRef] [PubMed] [Google Scholar]
- Ghosh S, Shankar S, Srivastava S, kumar S, Kumar A, Nagar G. et al. In vitro acaricidal properties of Semecarpus anacardium fruit and Datura stramonium leaf extracts against acaricide susceptible (IVRI-I line) and resistant (IVRI-V line) Rhipicephalus (Boophilus) microplus. Res Vet Sci 2015; 101:69-74. https://doi.org/10.1016/j.rvsc.2015.05.015 [CrossRef] [PubMed] [Google Scholar]
- Estrela AB, Seixas A, Teixeira O, Pinto AF, Termignoni C. Vitellin- and hemoglobin-digesting enzymes in Rhipicephalus (Boophilus) microplus larvae and females. Comp Biochem Physiol B Biochem Mol Biol 2010; 157(4):326-335. https://doi.org/10.1016/j.cbpb.2010.08.002 [CrossRef] [PubMed] [Google Scholar]
- Fagotto F. Yolk degradation in tick eggs: I Ocurrence of Cathepsin L-like acid proteinase in yolk spheres. Arch Insect Biochem Physiol 1990; 14(4):217-235.
- https://doi.org/10.1002/arch.940140403 [CrossRef] [PubMed] [Google Scholar]
- Rodríguez MCE, Pulido SNJ. Eficacia de extracts vegetales sobre la garrapata adulta Rhipicephalus (Boophilus) microplus y su oviposición. Rev Cubana Plant Med 2015; 20(4):375-388. http://www.revplantasmedicinales.sld.cu/index.php/pla/article/view/230 [Google Scholar]
- Brandão MGL, Zanetti NNS, Oliveira P, Grael CFF, Santos ACP, Monte-Mór RL. Brazilian medicinal plants described by 19th century European naturalists in the Official Pharmacopoeia. J Ethnopharmacol 2008; 120(2):141-148. https://doi.org/10.1016/j.jep.2008.08.004 [CrossRef] [PubMed] [Google Scholar]
- Ribeiro VL, Avancini C, Gonçalves K, Toigo E, von Poser G. Acaricidal activity of Calea serrata (Asteraceae) on Boophilus microplus and Rhipicephalus sanguineus. Vet Parasitol 2008; 151(2-4):351-354. https://doi.org/10.1016/j.vetpar.2007.11.007
- [CrossRef] [PubMed] [Google Scholar]























