Considerations for extracting blood from turtles and tortoises: venipuncture sites and anticoagulants
Consideraciones para la obtención de sangre en tortugas: sitios de venopunción y anticoagulantes

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
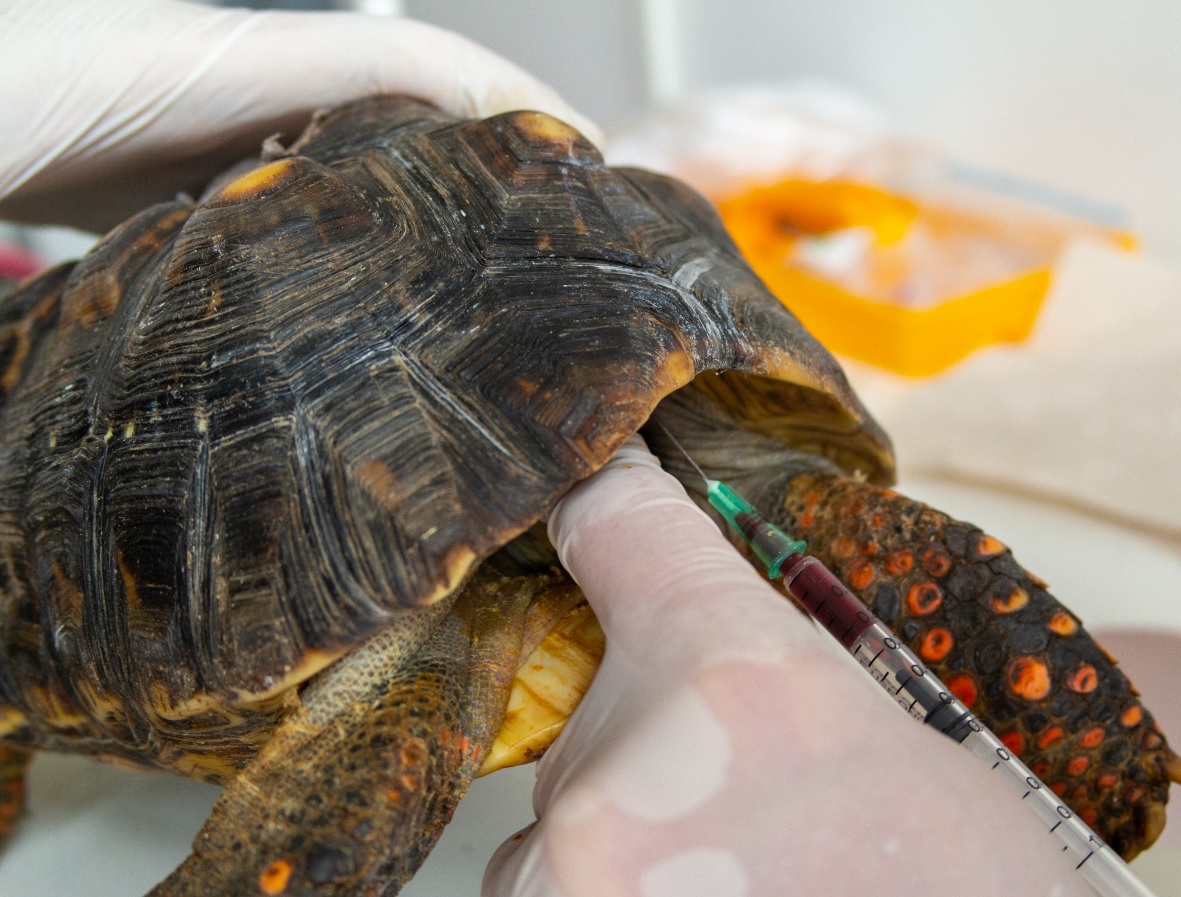
Objective. Evaluate different venipuncture points and the use of two anticoagulants to obtain blood samples in turtles. Materials and methods. Eighty-two turtles of the species Trachemys callirostris, Podocnemis unifilis and Chelonoidis carbonaria were sampled. Three venipuncture points were evaluated: subcarapacial venous sinus, dorsal coccygeal vein, and jugular vein. Two anticoagulants were tested: sodium heparin and EDTA. Results. The jugular vein was the best place to practice venipuncture as the blood samples obtained were free of hemodilution and enough volume to carry out a blood profile. In contrast, samples from the other venipuncture points were usually hemodiluted. Blood samples from C. carbonaria stored with EDTA (40 µl/ml of blood) showed haemolisis, which was not observed using sodium heparin (100 UI/ml of blood) as anticoagulant. Conclusions. The jugular vein is the most recommended venipuncture site for the extraction of blood samples for clinical purposes. Sodium heparin was the best anticoagulant to store blood samples due to the fact that it does not induce haemolisis in any sample.
Article visits 1421 | PDF visits
Downloads
- Moreno LA, Andrade GI, Ruíz-Contreras LF. Biodiversidad 2016. Estado y tendencias de la biodiversidad continental de Colombia. Bogotá, Colombia: Instituto de Investigación de Recursos Biológicos Alexander von Humboldt; 2016. http://repository.humboldt.org.co//handle/20.500.11761/32962
- Gibbons JW, Scott DE, Ryan TJ, Buhlmann KA, Tuberville T, Metts BS, et al. The Global Decline of Reptiles, Déjà Vu Amphibians. Bioscience. 2000; 50(8):653–66. https://doi.org/10.1641/0006-3568(2000)050[0653:TGDORD]2.0.CO;2
- Hofmeyr MD, Henen BT, Walton S. Season, sex and age variation in the haematology and body condition of geometric tortoises Psammobates geometricus. African Zool. 2017; 52(1):21–30. https://doi.org/10.1080/15627020.2017.1284575
- Arcila VH. Hematología y química sérica en hembras quelonios (Trachemys scripta callirostris) en la ribera del río Lebrija, Puerto Wilches (Santander) Parte I. Spei Domus. 2005; 1(2). https://revistas.ucc.edu.co/index.php/sp/article/view/568
- Carrascal J, Negrete H, Rojano C, Álvarez G, Chacón J, Linares J. Caracterización hematológica de hicoteas (Trachemys callirostris Gray, 1856) en Córdoba, Colombia. Rev Med Vet. 2014; 28:43. https://doi.org/10.19052/mv.3180
- Oliveira-Júnior AA, Tavares-Dias M, Marcon JL. Biochemical and hematological reference ranges for Amazon freshwater turtle, Podocnemis expansa (Reptilia: Pelomedusidae), with morphologic assessment of blood cells. Res Vet Sci. 2009; 86(1):146–151. https://doi.org/10.1016/j.rvsc.2008.05.015
- Rossini M, Blanco PA, Marín E, Comerma-Steffensen S, Zerpa H. Haematological values of post-laying Arrau turtle (Podocnemis expansa) in the Orinoco River, Venezuela. Res Vet Sci. 2012; 92(1):128–131. https://doi.org/10.1016/j.rvsc.2010.10.026
- Rojas G, Varillas L. Hemograma de la Tortuga Taricaya (Podocnemis unifilis). Hosp Vet. 2013; 5(1):13–15. http://www.rhv.cl/index.php?option=com_docman&task=doc_download&gid=67&Itemid=
- Ferronato BO, Genoy-puerto A, Piña CI, Souza FL, Verdade LM, Matushima ER. Notes on the hematology of free-living Phrynops geoffroanus (Testudines: Chelidae) in polluted rivers of Southeastern Brazil. Zool. 2009; 26(4):795–798. https://doi.org/10.1590/S1984-46702009000400027
- Muñoz-Pérez JP, Lewbart GA, Hirschfeld M, Alarcón-Ruales D, Denkinger J, Castañeda JG, et al. Blood gases, biochemistry and haematology of Galápagos hawksbill turtles (Eretmochelys imbricata). Conserv Physiol. 2017; 5(1). https://doi.org/10.1093/conphys/cox028
- Lewbart GA, Griffioen JA, Savo A, Muñoz-Pérez JP, Ortega C, Loyola A, et al. Biochemistry and hematology parameters of the San Cristóbal Galápagos tortoise (Chelonoidis chathamensis). Conserv Physiol. 2018; 6(1). https://doi.org/10.1093/conphys/coy004
- Cabrera M, Li O, Gálvez H, Sánchez N, Rojas G. Valores hematológicos de la tortuga motelo (Geochelone denticulata) mantenida en cautiverio. Rev Investig Vet del Peru. 2011; 22(2):144–150. https://doi.org/10.15381/rivep.v22i2.287
- Naguib M. How to take blood from a tortoise. Companion Anim. 2016; 21(7):422–425. https://doi.org/10.12968/coan.2016.21.7.422
- Crawshaw GJ, Holz P. Comparison of Plasma Biochemical Values in Blood and Blood-Lymph Mixtures from Red-eared Sliders, Trachemys scripta elegans. Bull Assoc Reptil Amphib Vet. 1996; 6(2):7–9. https://doi.org/10.5818/1076-3139.6.2.7
- Muro J, Cuenca R, Pastor J, Vinas L, Lavin S. Effects of Lithium Heparin and Tripotassium EDTA on Hematologic Values of Hermann’s Tortoises (Testudo hermanni). J Zoo Wildl Med. 1998; 29(1):40–44. https://www.jstor.org/stable/20095714
- Perpiñán D. Chelonian haematology: 1. Collection and handling of samples. In Pract. 2017; 39(5):194–202. https://doi.org/10.1136/inp.j1692
- Innis C, Knotek Z. Tortoises and Freshwater Turtles. En: Heatley J, Russell K, editors. Exotic Animal Laboratory Diagnosis. 1° ed. Hoboken, USA: Wiley; 2020. https://doi.org/10.1002/9781119108610.ch16
- Redrobe S, MacDonald J. Sample Collection and Clinical Pathology of Reptiles. Vet Clin North Am Exot Anim Pract. 1999; 2(3):709–730. https://doi.org/10.1016/S1094-9194(17)30118-4
- Mans C. Venipuncture techniques in chelonian species. Lab Anim. 2008; 37(7):303–304. https://doi.org/10.1038/laban0708-303
- Hattingh J, Smith EM. Anticoagulants for avian and reptilian blood: Heparin and EDTA. Pflügers Arch Eur J Physiol. 1976; 363(3):267–269. https://doi.org/10.1007/BF00594613
- Natt MP, Herrick CA. A New Blood Diluent for Counting the Erythrocytes and Leucocytes of the Chicken. Poult Sci. 1952; 31(4):735–738. https://doi.org/10.3382/ps.0310735
- Johnson RD, Nielsen CL. Traumatic Amputation of Finger From an Alligator Snapping Turtle Bite. Wilderness Environ Med. 2016; 27(2):277–281. https://doi.org/10.1016/j.wem.2016.02.003
- Gottdenker NL, Jacobson ER. Effect of venipuncture sites on hematologic and clinical biochemical values in desert tortoises (Gopherus agassizii). Am J Vet Res. 1995; 56(1):19–21.
- Eatwell K, Hedley J, Barron R. Reptile haematology and biochemistry. In Pract. 2014; 36(1):34–42. https://doi.org/10.1136/inp.f7488
- López-Olvera JR, Montané J, Marco I, Martínez-Silvestre A, Soler J, Lavin S. Effect of venipuncture site on hematologic and serum biochemical parameters in marginated tortoise (Testudo marginata). J Wildl Dis. 2003; 39(4):830–836. https://doi.org/10.7589/0090-3558-39.4.830
- Medeiros N, Locatelli-dittrich R, Schmidt E, Alvares A, Patrício L, Lange RR, et al. Efeito do sítio de venopunção nos parâmetros hematológicos em tigre-d’água-americano, Trachemys scripta elegans. Pesqui Vet Bras. 2012; 32(1):37–40. http://pvb.org.br/portal/download_artigo/MTA1MHwyMDIxMDMwNDE5NTk1NA==
- Perpiñán D, Armstrong DL, Dórea F. Effect of Anticoagulant and Venipuncture Site on Hematology and Serum Chemistries of the Spiny Softshell Turtle (Apalone spinifera). J Herpetol Med Surg. 2011; 20(2–3):74–78. https://doi.org/10.5818/1529-9651-20.2.74
- Lyman RA. The anti-haemolytic function of calcium in the blood of the snapping turtle, Chelydra serpentina. J Cell Comp Physiol. 1945; 25(1):65–73. https://doi.org/10.1002/jcp.1030250108
- Gradela A, Souza VN, Queiroz MM de, Constantino A da C, Bandeira CGC, Faria MD de, et al. Biometria corporal e parâmetros hematológicos de Trachemys scripta elegans e Trachemys dorbignyi (Testudines: Emydidae) criadas em cativeiro em Petrolina, Pernambuco. Pesqui Vet Bras. 2017; 37(1):83–90. https://doi.org/10.1590/s0100-736x2017000100014
- Kakizoe Y, Sakaoka K, Kakizoe F, Yoshii M, Nakamura H, Kanou Y, et al. Successive changes of hematologic characteristics and plasma chemistry values of juvenile loggerhead turtles (Caretta caretta). J Zoo Wildl Med. 2007; 38(1):77–84. https://doi.org/10.1638/05-096.1
- Rohilla MS, Tiwari PK. Simple method of blood sampling from Indian freshwater turtles for genetic studies. Acta Herpetológica. 2008; 3(1):65–69. https://doi.org/10.13128/Acta_Herpetol-2485
- Zaias J, Norton T, Fickel A, Spratt J, Altman NH, Cray C. Biochemical and hematologic values for 18 clinically healthy radiated tortoises (Geochelone radiata) on St Catherines Island, Georgia. Vet Clin Pathol. 2006; 35(3):321–325. https://doi.org/10.1111/j.1939-165X.2006.tb00139.x
- García GC, Alves‐Júnior JRF, Santana ÁE, Stas CMF, Silva CC, Kanayama CY, et al. Hematologic variables of the Arrau turtle (Podocnemis expansa) under the effects of different anticoagulants and cytologic stains. Vet Clin Pathol. 2021; 50(2):209–215. https://doi.org/10.1111/vcp.12960























