First evidence of Cichlidogyrus Paperna, 1960 (Monogenea: Ancyrocephalidae) in Oreochromis spp. cultures from Ecuador
Primera evidencia de Cichlidogyrus Paperna, 1960 (Monogenea: Ancyrocephalidae) en cultivos de Oreochromis spp. de Ecuador

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
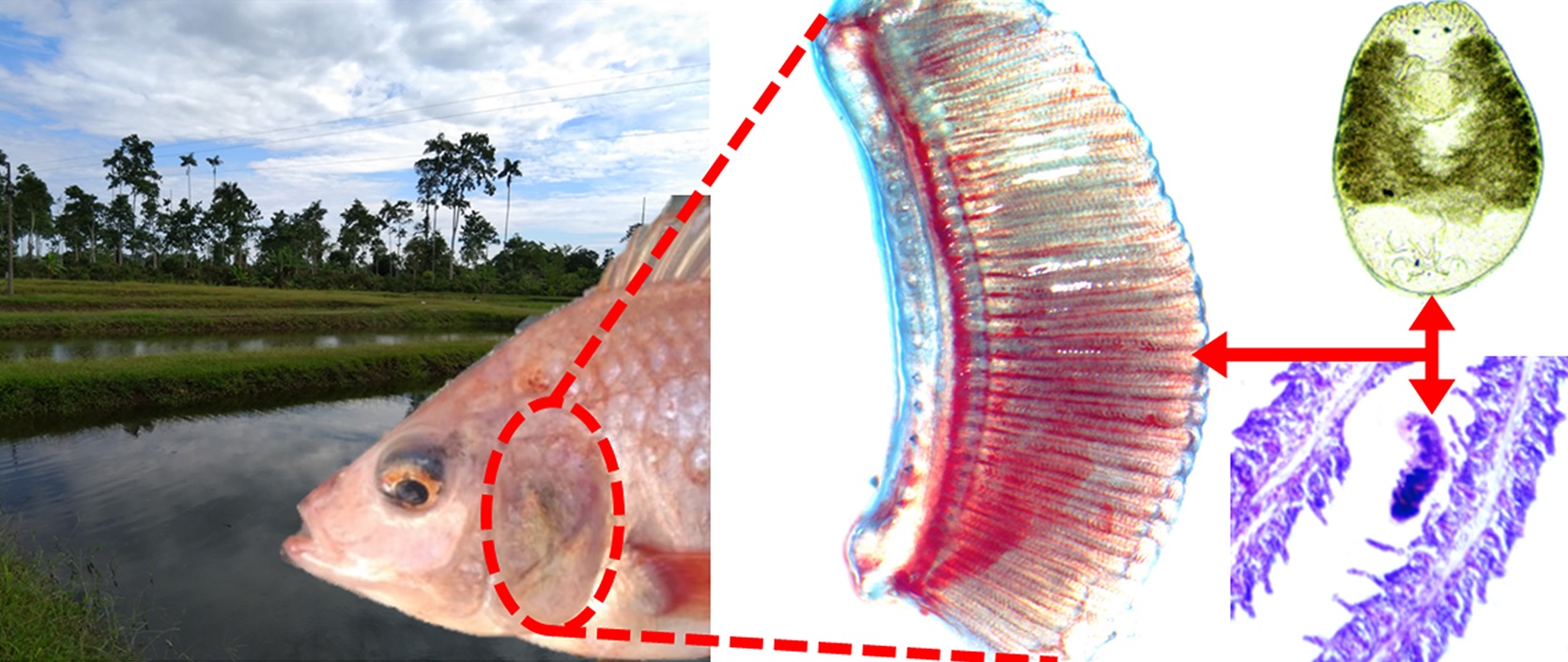
Objective. To evidence of the presence of two species of monogeneans of the genus Cichlidogyrus that cause damage to gill tissue, in tilapia Oreochromis spp. grown in Ecuador. Materials and methods. The gills were placed in a Petri dish, boiling water was added to it so that the parasites relax, and later they were fixed in 4% formalin. The samples were checked under a stereoscope microscope. The parasites were counted and preserved in 70% ethanol for their taxonomic identification according to their morphological characteristics. The infection parameters (prevalence, mean abundance and mean intensity) were calculated for each species of parasite. For the description of histological damage, fragments of parasitized gills were fixed in 10% neutral formalin and processed by the histological paraffin embedding technique. Results. Cichlidogyrus sclerosus showed highest infection parameters, prevalence of 65%, mean abundance of 24.24 ± 68.62 and mean intensity of 37.62 ± 82.93. Cychlidogyrus dossoui was observed in a prevalence of 4.5%, mean abundance of 0.13 ± 0.66 and mean intensity of 3 ± 1.41. Both species caused hyperplasia in lamellae. Conclusions. This study constitutes the first report of infestation by Cichlidogyrus sclerosus and C. dossoui in tilapia culture systems of Ecuador.
Article visits 532 | PDF visits
Downloads
- Wilson JR, Saunders RJ, Hutson KS. Parasites of the invasive tilapia Oreochromis mossambicus: evidence for co-introduction. Aquat Invasions. 2019; 14(2):332–349. https://www.reabic.net/aquaticinvasions/2019/AI_2019_Wilson_etal.pdf
- Paredes-Trujillo A, Velázquez-Abunader I, Torres-Irineo E, Romero D, Vidal-Martínez VM. Geographical distribution of protozoan and metazoan parasites of farmed Nile tilapia Oreochromis niloticus (L.) (Perciformes: Cichlidae) in Yucatán, Mexico. Parasit Vectors. 2016; 9(66). https://doi.org/10.1186/s13071-016-1332-9
- Soler-Jiménez LC, Paredes-Trujillo AI, Vidal-Martínez VM. Helminth parasites of finfish commercial aquaculture in Latin America. J Helminthol. 2017; 91(2):110–136. https://doi.org/10.1017/S0022149X16000833
- Gonzales-Fernández JG. Parasitofauna of tilapia cause mortalities in fingerlings in two fishfarms, Lima, Peru. Neotrop Helminthol. 2012; 6(2):219–229. https://sisbib.unmsm.edu.pe/BVRevistas/neohel/v6n2/pdf/a08v6n2.pdf
- Jacome J, Quezada C, Sánchez O, Pérez JE, Nirchio M. Tilapia en Ecuador: paradoja entre la producción acuícola y la protección de la biodiversidad ecuatoriana. Rev peru de biol. 2019; 26(4):543–550. http://dx.doi.org/10.15381/rpb.v26i4.16343
- FAO. (2020). Fisheries Division, Statistics and information Branch. FishStatJ: Universal software for fishery statistical time series. Copyright 2020. http://www.fao.org/fishery/statistics/software/fishstatj/es
- Pariselle A, Euzet L. Systematic revision of dactylogyridean parasites (Monogenea)from cichlid fishes in Africa, the Levant and Madagascar. Zoosystema 2009; 31(4):849-898. https://doi.org/10.5252/z2009n4a6
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology Meets Ecology on Its Own Terms: Margolis et al. Revisited. J Parasitol. 1997; 83(4):575-583. https://www.doi.org/10.2307/3284227
- Wolf J, Baumgartner W, Blazer V, Camus AC, Engelhardt JA, Fournie JW, et al. Non-lesions, misdiagnoses, missed diagnoses, and other interpretive challenges in fish histopathology studies: a guide for investigators, authors, reviewers, and readers. Toxicol Pathol. 2014; 43: 297–325. https://www.doi.org/10.1177/0192623314540229
- Steckert LD, Cardoso L, Jerônimo GT, Benites S, Martins ML. Investigation of farmed Nile tilapia health through histopathology. Aquaculture. 2018; 486:161–169. https://www.doi.org/10.1016/j.aquaculture.2017.12.021
- Douellou L. Monogeneans of the genus Cichlidogyrus Paperna, 1960 (Dactylogyridae: Ancyrocephalinae) from cichlid fishes of Lake Kariba (Zimbabwe) with descriptions of five new species. Syst Parasitol. 1993; 25:159–186. https://www.doi.org/10.1007/BF00007007
- Madanire-Moyo GN, Matla MM, Olivier PAS, Luus-Powell WJ. Population dynamics and spatial distribution of monogeneans on the gills of Oreochromis mossambicus (Peters, 1852) from two lakes of the Limpopo River System, South Africa. J Helminthol. 2011; 85(2):146–152. https://www.doi.org/10.1017/S0022149X10000301
- Madanire-Moyo GN, Luus-Powell WJ, Olivier PA. Diversity of metazoan parasites of the Mozambique tilapia, Oreochromis mossambicus (Peters, 1852), as indicators of pollution in the Limpopo and Olifants river systems. OJVR. 2012; 79:1–9. https://doi.org/10.4102/ojvr.v79i1.362
- Pariselle A, Bitja Nyom AR, Bilong Bilong C. Checklist of the ancyrocephalids (Monogenea) parasitizing Tilapia species in Cameroon, with the description of three new species. Zootaxa. 2013; 3599, 78–86. https://doi.org/10.11646/zootaxa.3599.1.7
- Fannes W, Vanhove MPM, Huyse T. Redescription of Cichlidogyrus tiberianus Paperna, 1960 and C. dossoui Douëllou, 1993 (Monogenea: Ancyrocephalidae), with special reference to the male copulatory organ. Syst Parasitol. 2017; 94(1):133–44. https://www.doi.org/10.1007/s11230-016-9685-1
- Paperna I, Thurston JP. (1969) Monogenetic trematodes collected from cichlid fish in Uganda; including the description of five new species of Cichlidogyrus. Rev Zool afric, 1969; 79(1-2):15-33. https://www.cabdirect.org/cabdirect/abstract/19700802719
- Aguirre-Fey D, Benítez-Villa GE, Pérez-Ponce de León G, Rubio-Godoy M. Population dynamics of Cichlidogyrus spp. and Scutogyrus sp. (Monogenea) infecting farmed tilapia in Veracruz, México. Aquaculture. 2015; 443:11–15. https://www.doi.org/10.1016/j.aquaculture.2015.03.004
- Maneepitaksanti W, Nagasawa K. Monogeneans of Cichlidogyrus Paperna, 1960 (Dactylogyridae), gill parasites of tilapias, from Okinawa Prefecture, Japan. Biogeography 2012; 14:111–119. https://ir.lib.hiroshima-u.ac.jp/files/public/3/34082/20141016200842209265/Biogeography_14_111.pdf
- Jiménez R. Enfermedades de tilapia en cultivo. Cámara Ecuatoriana del Libro, Núcleo de Pichincha; 2007. https://isbn.cloud/9789942010735/enfermedades-de-tilapia-en-cultivo/
- Kritsky DC, Thatcher VE. Monogenetic trematodes (Monopisthocotylea: Dactylogyridae) from freshwater fishes of Colombia, South America. J Helminthol, 1974; 48:59-66. https://doi.org/10.1017/S0022149X00022604
- Messu Mandeng FDM, Bilong Bilong CF, Pariselle A, Vanhove MPM, Bitja Nyom AR, Agnèse J-F. A phylogeny of Cichlidogyrus spp. (Monogenea, Dactylogyridae) clarifies a host-switch between fish families and reveals an adaptive component to attachment organ morphology of this parasite genus. Parasit Vectors. 2015; 8:582. https://doi.org/10.1186/s13071-015-1181-y
- Vignon M, Pariselle A, Vanhove MPM. Modulatory in attachment organs of African Cichlidogyrus (Platyhelminthes: Monogenea: Ancyrocephalidae) reflects phylogeny rather tan host specificity or geografic distribution. Biol J Linn Soc. 2011; 102:694-706. https://doi.org/10.1111/j.1095-8312.2010.01607.x
- Ojwala RA, Otachi EO, Kitaka NK. Effect of water quality on the parasite assemblages infecting Nile tilapia in selected fish farms in Nakuru County, Kenya. Parasitol Res. 2018; 117(11):3459-3471. https://doi.org/10.1007/s00436-018-6042-0
- Akoll P, Fioravanti ML, Konecny R, Schiemer F. Infection dynamics of Cichlidogyrus tilapiae and C. sclerosus (Monogenea, Ancyrocephalinae) in Nile tilapia (Oreochromis niloticus L) from Uganda. J Helminthol. 2011; 86(3):302-310. https://doi.org/10.1017/S0022149X11000411
- Ibrahim MM. Variation in parasite infracommunies of Tilapia zillii in relation to some biotic and abiotic factors. Int J Zool. 2012; 8(2):59-70. https://doi.org/10.3923/ijzr.2012.59.70
- Paredes-Trujillo A, Velázquez-Abunader I, Papiol V, del Rio-Rodríguez RE, Vidal-Martínez VM. Negative effect of ectoparasite burdens on the condition factor from farmed tilapia Oreochromis niloticus in the Yucatan, Mexico. Vet Parasitol. 2021; 292:109393. https://www.doi.org/10.1016/j.vetpar.2021.109393
- Igeh PC, Avenant-Oldewage A. Pathological effects of Cichlidogyrus philander Douëllou, 1993 (Monogenea, Ancyrocephalidae) on the gills of Pseudocrenilabrus philander (Weber, 1897) (Cichlidae). J Fish Dis. 2020; 43(2):177–184. https://www.doi.org/10.1111/jfd.13121
- Martins ML, Cardoso L, Marchiori N, Benites de Pádua S. Protozoan infections in farmed fish from Brazil: diagnosis and pathogenesis. Rev. Bras Parasitol Vet. 2015; 24(1):1–20. https://doi.org/10.1590/S1984-29612015013
- El-Mansy A, Hamada S, Hasan S, El-Sarnagawy D. Histopathology of farmed freshwater fish infested with different helminthes. Egypt J Aquat Biol & Fish. 2011; 15(1):1-13. https://doi.org/10.21608/EJABF.2011.2072
- Alim DIM, Matter HMA. Histopathological alteration induced in gills of juvenile Nile tilapia Oreochromis niloticus upon exposure to two bio-pesticides. Int J Fish Aquat. Stud. 2015; 2(5):80–83. https://www. fisheriesjournal.com/archives/?year=201 5&vol=2&issue=5&part=B&ArticleId=454























