Examination of adenoviruses with molecular and pathological methods in sheep pneumonia cases
Examen de adenovirus con métodos moleculares y patológicos en casos de pneumonía ovina

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
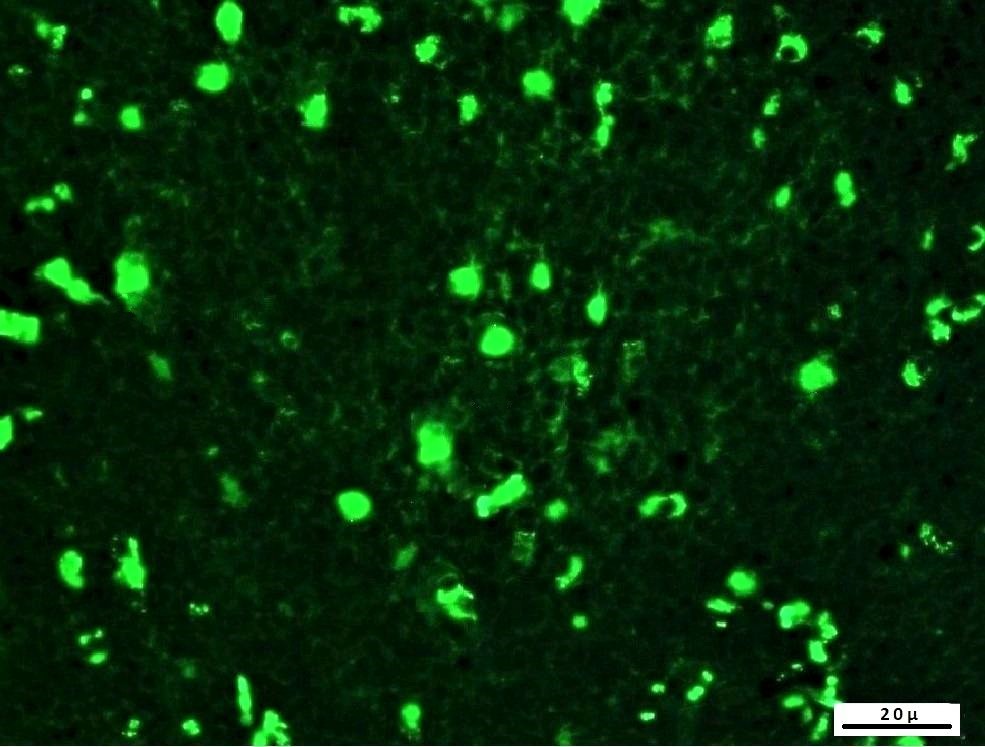
Objective. Reveal adenoviruses (AdV) that cause pneumonia in sheep and examine pathologic changes in the pulmonary and mediastinal lymph nodes of naturally infected adenovirus-positive specimens. Material and method. For this purpose, 1459 lungs of sheep slaughtered in a slaughterhouse were macroscopically examined, and pneumonia lesions were detected in 88 (6.03%) of these. The paraffinized tissue sections of these specimens with pneumonia were examined with the immunohistochemical (IHC) and indirect immunofluorescence (IF) methods, whereas their tissue homogenates were examined using the Antigen ELISA and PCR methods for adenovirus positivity. Results. Accordingly, the prevalence of adenoviruses was determined as 19.3% for IHC, 22.7% for IF, 20.5% for ELISA and 13.6% for PCR. Hematoxylin-eosin (HE) staining was performed to examine histopathological changes in the specimens that were naturally infected with adenoviruses. The histopathological examinations of the naturally infected lung specimens revealed mainly interstitial pneumonia, as well as catarrhal and verminous pneumonia findings. Consequently, it was determined that the most effective methods in the detection of adenoviruses in sheep pneumonias were found respectively as IF, ELISA, IHC and PCR. The finding that adenoviruses were observed only in the mediastinal lymph nodes of some specimens in the immunopathological methods suggested that the latency. Conclusions. The presence of adenoviruses in sheep pneumonia cases was determined with the indirect immunofluorescence, antigen ELISA and PCR methods for the first time. The possibility of the latent nature of adenovirus infection in these species was also discussed for the first time.
Article visits 414 | PDF visits
Downloads
- Saleh NS, Allam TS. Pneumonia in sheep: bacteriological and clinicopathological studies. Am J Res Commun.2014; 2(11):70-88. http://www.usa-journals.com/wp-content/uploads/2014/10/Saleh_Vol211.pdf
- McRae KM, Baird HJ, Dodds KG, Bixley MJ, Clarke SM. Incidence and heritability of ovine pneumonia, and the relationship with production traits in New Zealand sheep. Small Rumin Res. 2016; 145:136-141. https://doi.org/10.1016/j.smallrumres.2016.11.003
- Watkiss ER. Pathogenesis of respiratory syncytial virus. Curr Opin Virol. 2012;2(3):300-305. https://doi.org/10.1016/j.coviro.2012.01.008
- Benavides J, González L, Dagleish M, Pérez V. Diagnostic pathology in microbial diseases of sheep or goats. Vet Microbiol. 2015; 181(1-2):15-26. https://doi.org/10.1016/j.vetmic.2015.07.012
- Benkő M, Aoki K, Arnberg N, Davison AJ, Echavarría M, Hess M, et al. ICTV Virus Taxonomy Profile: Adenoviridae 2022. J Gen Virol. 2022; 103(3):001721. https://doi.org/10.1099/jgv.0.001721
- Debey BM, Lehmkuhl HD, Chard-Bergstrom C, Hobbs LA. Ovine adenovirus serotype 7-associated mortality in lambs in the United States. Vet Pathol. 2001; 38(6):644-648. https://doi.org/10.1354/vp.38-6-644
- Rahal A, Ahmad AH, Prakash A, Mandil R, Kumar AT. Environmental attributes to respiratory diseases of small ruminants. Vet Med Int. 2014; 2014:1-10. https://doi.org/10.1155/2014/853627
- Kumar MA, Kumar R, Varshney KC, Nair MG, Lakkawar AW, Sridhar BG, Palanivelu, M. Pathomorphological studies of lung lesions in sheep. Indian J Vet Pathol. 2014; 38(2):75-81. https://doi.org/10.5958/0973-970X.2014.01142.0
- Giberson AN, Davidson AR, Parks RJ. Chromatin structure of adenovirus DNA throughout infection. Nucleic Acids Res. 2012; 40(6):2369-2376. https://doi.org/10.1093/nar/gkr1076
- Greber UF, Flatt JW. Adenovirus entry: from infection to immunity. Annu Rev Virol. 2019; 6:177-197. https://doi.org/10.1146/annurev-virology-092818-015550
- Andres CJ, Angelica ÁM, David CJ. Enfermedades respiratorias de vías aéreas bajas en ovinos, impacto regional, principales etiologías infecciosas y métodos de diagnóstico. Rev. Zooc. 2016; 3(1):25-32. https://revistas.udca.edu.co/index.php/zoociencia/article/view/522
- Matthes-Martin S, Boztug H, Lion T. Diagnosis and treatment of adenovirus infection in immunocompromised patients. Expert Rev Anti Infect Ther. 2013; 11(10):1017-1028. https://doi.org/10.1586/14787210.2013.836964
- Huang HS, Tsai CL, Chang J, Hsu TC, Lin S, Lee CC. Multiplex PCR system for the rapid diagnosis of respiratory virus infection: systematic review and meta-analysis. Clin Microbiol Infect. 2018; 24(10):1055-1063. https://doi.org/10.1016/j.cmi.2017.11.018
- Chakraborty S, Kumar A, Tiwari R, Rahal A, Malik Y, Dhama K, et al. Advances in diagnosis of respiratory diseases of small ruminants. Vet Med Int. 2014; 2014,1-16. http://dx.doi.org/10.1155/2014/508304
- Abd El-Ghany WA. A Comprehensive Review on Adenoviruses Infections in Fowl: Epidemiology, Forms, Diagnosis, and Control. J World's Poult Res. 2021; 11(2):151-167. https://dx.doi.org/10.36380/jwpr.2021.19
- Sibley SD, Goldberg TL, Pedersen JA. Detection of known and novel adenoviruses in cattle wastes via broad-spectrum primers. Appl Environ Microbiol. 2011; 77(14):5001-5008. https://doi.org/10.1128/AEM.00625-11
- Minakshi P, Ranjan K, Brar B, Ambawat S, Shafiq M, Alisha A, et al. New approaches for diagnosis of viral diseases in animals. Adv Anim Vet Sci. 2014; 2(4S):55-63. http://dx.doi.org/10.14737/journal.aavs/2014/2.4s.55.63
- Davies DH, Dungworth DL, Mariassy AT. Experimental adenovirus infection of lambs. Vet Microbiol. 1981; 6(2):113-128. https://doi.org/10.1016/0378-1135(81)90004-3
- Sharp JM, Rushton B, Rimer RD. Experimental infection of specific pathogen-free lambs with ovine adenovirus type 4. J Comp Pathol. 1976; 86(4):621-628. https://doi.org/10.1016/0021-9975(76)90071-2
- Çeribasi AO, Çeribasi S, Ozkaraca M. Immunohistochemical detection of bovine herpesvirus type 1 and bovine adenovirus type 3 antigens in frozen and paraffinized lung sections of pneumonic sheep and goats. Vet Arh. 2016; 86(1):9-21. http://wwwi.vef.hr/vetarhiv/papers/2016-86-1-2.pdf
- Jamshidi K, Ozmen O, Rahmani M, Marvaki R, Soltanmohammadi M. Adenovirus Antigen Detection in Paraffinized Lung Sections of Pneumonic Goat Lungs Using Immunohistochemistry. Iran J Vet Res. 2019; 13(2):143-150.https://doi.org/10.22059/IJVM.2019.262877.1004913
- Borujeni MP, Hajikolaei MRH, Shapouri MRSA, Roshani F. The role of sheep in the epidemiology of Bovine alphaherpesvirus 1 (BoHV-1). Prev Vet Med. 2020; 174:104818. https://doi.org/10.1016/j.prevetmed.2019.104818
- Alpay G, Öner EB, Yeşilbağ K. Seroepidemiology and molecular investigation of pestiviruses among sheep and goats in Northwest Anatolia. Turk J Vet Anim Sci. 2018; 42(3):205-210. https://doi.org/10.1007/s11250-008-9225-3
- Alpay G, Tuncer P, Yeşilbağ K. Serological distribution of some viral infections in cattle, sheep and goats in an isolated island-ecosystem. Ankara Univ Vet Fak Derg. 2014; 61(1):43-48. https://doi.org/10.1501/Vetfak_0000002603
- Comakli S, Sağlam YS, Timurkan MÖ. Comparative detection of bovine herpesvirus-1 using antigen ELISA, immunohistochemistry and immunofluorescence methods in cattle with pneumonia. Turkish J Vet Anim Sci. 2019; 43(3):306-313. https://dergipark.org.tr/tr/download/article-file/734737
- Slatko BE, Gardner AF, Ausubel FM. Overview of next‐generation sequencing technologies. Curr Protoc Mol Biol. 2018; 122(1):e59. https://doi.org/10.1002/cpmb.59
- Hoeben RC, Uil TG. Adenovirus DNA replication. Cold Spring Harb Perspect Biol. 2013; 5(3):a013003. https://cshperspectives.cshlp.org/content/5/3/a013003
- González-López JJ, Morcillo-Laiz R, Muñoz-Negrete FJ. Adenoviral keratoconjunctivitis: an update. Arch Soc Esp Oftalmol. 2013; 88(3):108-115. https://doi.org/10.1016/j.oftale.2012.07.002
- Kundu A, McBride G, Wuertz S. Adenovirus-associated health risks for recreational activities in a multi-use coastal watershed based on site-specific quantitative microbial risk assessment. Water Res. 2013; 47(16):6309-6325. https://doi.org/10.1016/j.watres.2013.08.002
- Palomino-Tapia V, Mitevski D, Inglis T, Van der Meer F, Abdul-Careem MF. Molecular Characterization of Hemorrhagic Enteritis Virus (HEV) Obtained from Clinical Samples in Western Canada 2017–2018. Viruses. 2020; 12(9):941. https://doi.org/10.3390/v12090941
- Wang H, Zheng Y, Deng J, Chen X, Liu P, Li X. Molecular epidemiology of respiratory adenovirus detection in hospitalized children in Shenzhen, China. Int J Clin Exp Med. 2015; 8(9):15011. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4658874























