Serum biochemistry and hematological profile of a cat with three mummified fetuses
Bioquímica sérica y perfil hematológico de una gata con tres fetos momificados

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
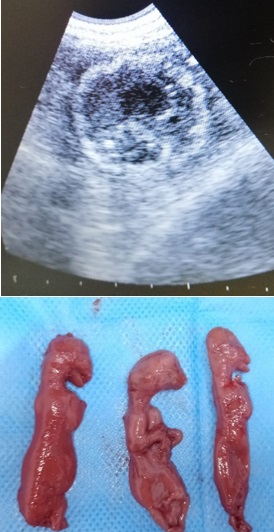
Serum biochemistry and hematological values are used to determine the outcome of diseases in both animals and humans. In the presented scientific report, hematological and biochemical findings were defined in the cat, which was shaped as three mummified fetuses. One old cat, which was mated 38 days ago, was brought to the private pet clinic with complaints of vomiting, anorexia, and polydipsia. After the preliminary clinical examination, it was observed in the ultrasonographic examination that the fetus had no heartbeat and the hyperechoic areas increased. The mummified fetus was diagnosed. Fetal mummification is occasional in cats and has been reported. Blood samples were taken for serum biochemistry and hematological analysis. Considering that serum biochemistry and hematological analyzes are important in cases of mummified fetuses in cats, this case report is presented. However, both hematological and biochemical parameters were within the reference ranges. Ovariohysterectomy was performed under general anesthesia. Seven days after the surgery, the wound from the operation was completely healed.
Article visits 364 | PDF visits
Downloads
- Johnston SD, Raksil S. Fetal loss in the dog and cat. Vet Clin North Am Small Anim Pract. 1987; 17(3):535-554. https://doi.org/10.1016/S0195-5616(87)50052-3
- Lamm CG, Njaa BL. Clinical approach to abortion, stillbirth, and neonatal death in dogs and cats. Vet Clin North Am Small Anim Pract. 2012; 42(3):501-513. https://doi.org/10.1016/j.cvsm.2012.01.015
- Lefebvre R. Fetal mummification in the major domestic species: current perspectives on causes and management. Vet Med. 2015; 6:233-244. https://www.dovepress.com/getfile.php?fileID=25364
- Gabor LJ, Canfield PJ, Malik R. Haematological and biochemical findings in cats in Australia with lymphosarcoma. Aust Vet J. 2006; 78:456-461. https://doi.org/10.1111/j.1751-0813.2000.tb11856.x
- Yamada T. Serum amyloid A (SAA): A concise review of biology, assay methods and clinical usefulness. Clin Chem Lab Med. 1999; 37:381-388. https://doi.org/10.1515/CCLM.1999.063
- Ceron JJ, Eckersall PD, Martynez-Subiela S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet Clin Pathol. 2005; 4:85-99. https://doi.org/10.1111/j.1939-165x.2005.tb00019.x
- Wycislo KL, Connolly SL, Slater MR, Makolinski KV. A biochemical survey of free-roaming cats (Felis catus) in New York City was presented to a trap–neuter–return program. J. Feline Med. Surg. 2014; 16(8):657-662. https://doi.org/10.1177/1098612X13517253
- Safak T, Yilmaz O, Ercan K, Yuksel B, Ocal H. A case of vaginal hyperplasia occurred the last trimester of pregnancy in a Kangal bitch. Ankara Univ Vet Fak Derg. 2021; 68(3):307-310. https://doi.org/10.33988/auvfd.764656
- Hossain A, Noor J, Yadav SK. Fetal mummification in a cat. Acta Scientific Vet Sci. 2021; 3(1):19-22. https://actascientific.com/ASVS/pdf/ASVS-03-0120.pdf
- Sabuncu A, Günay Z, Uçmak M, Enginler SÖ, Erzengin ÖM, Kurban I, Kahraman BB. Different sizes and degrees of foetal mummification during pregnancy in a dog: a case report. Inter J. Vet. Sci. 2013; 2(2):75-77. http://www.ijvets.com/pdf-files/Volume-2-no-2-2013/75-77.pdf
- Kajikawa T, Furuta A, Onishi T, Tajima T, Sugii S. Changes in concentrations of serum amyloid A protein, a1-acid glycoprotein, haptoglobin, and C-reactive protein in feline sera due to induced inflammation and surgery. Vet. Immunol. Immunopathol. 1999; 68:91–98. https://doi.org/10.1016/s0165-2427(99)00012-4
- Korman RM, Ceron JJ, Knowles TG, Barker EN, Eckersall PD, Tasker S. Acute phase response to Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ infection in FIV-infected and non-FIV-infected cats. Vet. J. 2012; 193:433–438. https://doi.org/10.1016/j.tvjl.2011.12.009
- Tamamoto T, Ohno K, Ohmi A, Seki I, Tsujimoto H. Time-course monitoring of serum amyloid A in a cat with pancreatitis. Vet Clin Pathol. 2009; 38:83–86. https://doi.org/10.1111/j.1939-165X.2008.00082.x
- Javard, R, Grimes C, Bau-Gaudreault L, Dunn M. Acute-phase proteins and iron status in cats with chronic kidney disease. J Vet Intern Med. 2017; 31:457–464. https://doi.org/10.1111/jvim.14661
- Winkel VM, Pavan TL, Wirthl VA, Alves AL, Lucas SR. Serum alpha-1 acid glycoprotein and serum amyloid A concentrations in cats receiving antineoplastic treatment for lymphoma. Am J Vet Res. 2015; 76:983–988. https://doi.org/10.2460/ajvr.76.11.983
- Tamamoto T, Ohno K, Goto-Koshino Y, Tsujimoto H. Serum amyloid A promotes invasion of feline mammary carcinoma cells. J Vet Med Sci. 2014; 76:1183–1188. https://doi.org/10.1292/jvms.14-0108
- Hazuchova K, Held S, Neiger R. Usefulness of acute phase proteins in differentiating between feline infectious peritonitis and other diseases in cats with body cavity effusions. J Feline Med Surg. 2017; 19:809–816. https://doi.org/10.1177/1098612X16658925
- Vilhena H, Figueiredo M, Cerón JJ, Pastor J, Miranda S, Craveiro H, Tvarijonaviciute A. Acute phase proteins and antioxidant responses in queens with pyometra. Theriogenology. 2018; 115:30-37. https://doi.org/10.1016/j.theriogenology.2018.04.010
- Troia R, Gruarin M, Foglia A, Agnoli C, Dondi F, Giunti M. Serum amyloid A in the diagnosis of feline sepsis. J Vet Diagn Investig. 2017; 29:856–859. https://doi.org/10.1177/1040638717722815
- Yuki M, Aoyama R, Nakagawa M, Hirano T, Naitoh E, Kainuma D. A clinical investigation on serum amyloid A concentration in client-owned healthy and diseased cats in a primary care animal hospital. Vet Sci. 2020; 7:1-9. https://doi:10.3390/vetsci7020045
- Kann RK, Seddon JM, Henning J, Meers J. Acute phase proteins in healthy and sick cats. Res Vet Sci. 2012; 93:649–654. https://doi.org/10.1016/j.rvsc.2011.11.007
- Hwang J, Gottdenker N, Min MS, Lee H, Chun MS. Evaluation of biochemical and hematological parameters and prevalence of selected pathogens in feral cats from urban and rural habitats in South Korea. J Feline Med Surg. 2016; 18(6):443-451. https://doi.org/10.1177/1098612X15587572
- Mooney SC, Hayes AA, Matus RE, MacEwen EG. Renal lymphoma in cats: 28 cases (1977-1984). J Am Vet Med. 1987; 191(11):1473-1477. https://pubmed.ncbi.nlm.nih.gov/3693001