Parvitaenia cochlearii (Cestoda: Gryporhynchidae) in a cultivation of Pacific fat sleeper Dormitator latifrons from Ecuador
Parvitaenia cochlearii (Cestoda: Gryporhynchidae) en cultivo de chame Dormitator latifrons en Ecuador

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
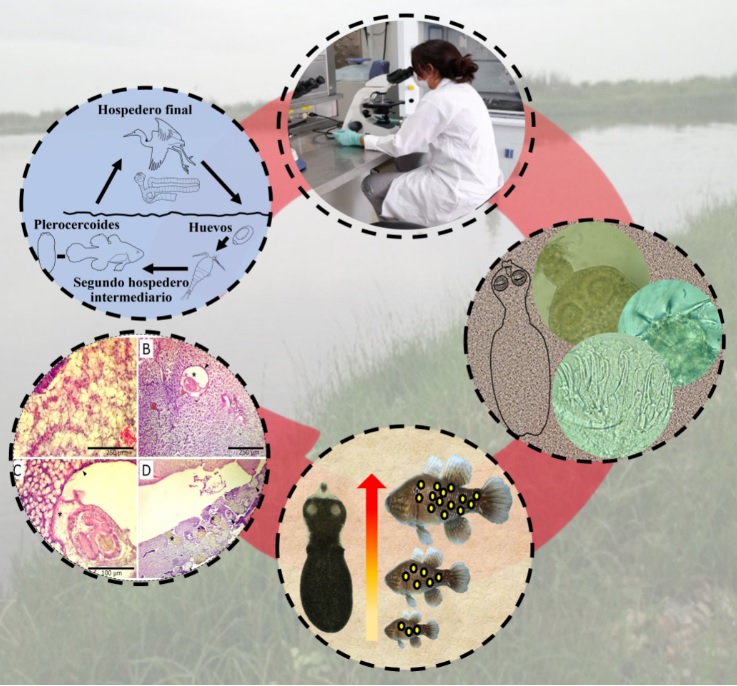
Objective. To identify and determine the infection parameters of the metacestodo Parvitaenia in different culture stages of chame Dormitator latifrons and to evaluate the histological damage caused by this parasite. Materials and methods. Forty-five specimens, 15 pre-breeding, 15 pre-fattening and 15 fattening, were examined to identify the parasite by conventional parasitology techniques; and the infection prevalence and average intensity parameters were calculated. Tissue damage was evaluated by fixing liver fragments and processed with the kerosene embedding technique. The difference between intensity per culture phase was evaluated by Kruskal-Wallis test. Total length, weight and Fulton's condition factor were correlated with intensity by linear correlation analysis. Results. A total of 29151 cestodes were found, identified as Parvitaenia cochlearii. The prevalence of the cestode was 100 % in all culture stages. The average intensity ranged from 22.4 to 1729.5 individuals and showed significant differences between culture phases (KW(2;42) = 36.34; p<0.001). Intensity showed a positive correlation with total fish length (r2= 0.45; p<0.05) and with weight (r2= 0.38; p<0.05). Conclusions. This is the first report of P. cochlearii in cultured D. latifrons. Our results suggest that there is a tendency for cestodes to accumulate during the growth of chames, with infection being higher in the larger sizes, which could have negative implications for trade.
Article visits 520 | PDF visits
Downloads
- Vega-Villasante F, Ruiz-González LE, Chong-Carrillo O, Basto-Rosales MER, Palma-Cancino DJ, Tintos-Gómez A, et al. Biology and use of the pacific fat sleeper Dormitator latifrons (Richardson, 1844): State of the art review. Lat Am J Aquat Res. 2021; 49(3):391–403. http://dx.doi.org/10.3856/vol49-issue3-fulltext-2637
- FAO. Fisheries Division, Statics and information Branch. FishStatJ: Universal software for fishery statistical time series. Copyright 2020. 2020. https://www.fao.org/fishery/es/topic/166235?lang=en
- Flores-Nava A. Aquaculture seed resources in Latin America: a regional synthesis. In: Assessment of freshwater fish seed resources for sustainable aquaculture. [Internet]. FAO, Rome. 2007. https://books.google.es/books?id=RTT3jgo5lU4C&lpg=PA91&ots=a0_4lqJ-KA&dq=Aquaculture%20seed%20resources%20in%20Latin%20America%3A%20a%20regional%20synthesis&lr&hl=es&pg=PP1#v=onepage&q=Aquaculture%20seed%20resources%20in%20Latin%20America%3A%20a%20regional%20synthesis&f=false
- Garrido-Olvera L, García-Prieto L, Mendoza-Garfias B. Helminth Parasites of the Pacific Fat Sleeper, Dormitator latifrons (Richardson, 1844) (Osteichthyes: Eleotridae) from Tres Palos Lagoon, Guerrero, Mexico. Am Midl Nat. 2004; 151(1):165–169. https://doi.org/10.1674/0003-0031(2004)151[0165:HPOTPF]2.0.CO;2
- Violante-González J, Rojas-Herrera A, Leopoldina Aguirre-Macedo M. Seasonal patterns in metazoan parasite community of the “Fat Sleeper” Dormitator latifrons (Pisces: Eleotridae) from Tres Palos Lagoon, Guerrero, Mexico. Rev Biol Trop. 2008; 56(3):1419–1427. https://www.scielo.sa.cr/pdf/rbt/v56n3/art34v56n3.pdf
- Díaz-Camacho SP, Cruz-Otero M del C, Zazueta-Ramos ML, Bojórquez-Contreras A, Sicairos-Félix J, Guzmán-Loreto R, et al. Identification of estuarine fish Dormitator latifrons as an intermediate host and Eleotris picta as a paratenic host for Gnathostoma binucleatum in Sinaloa, Mexico. Parasitol Res. 2008; 103:1421–1425. https://doi.org/10.1007/s00436-008-1151-9
- Vega-Villasante F, Cueto-Cortes L, Basto-Rosales MER, Badillo-Zapata D, Chong-Carrillo O, Ruiz-González LE, et al. Presencia de Argulus sp. en un cultivo de Dormitator latifrons: prevalencia, mortalidad y tratamiento. Rev Bio Ciencias. 2017; 4(6):1–14. https://doi.org/10.15741/revbio.04.06.05
- Scholz T, Salgado-Maldonado G. Metacestodes of the family Dilepididae (Cestoda : Cyclophyllidea) parasitising fishes in Mexico. Syst Parasitol. 2001; 49:23–40. https://link.springer.com/article/10.1023/A:1010603732525
- Salgado-Maldonado G, Cabañas-Carranza, G. Caspeta-Mandujano, J. M. Soto-Galera E, Mayén-Peña E, Brailovsky D, Báez-Valé R. Helminth parasites of freshwater fishes of the Balsas River drainage basin of the southwestern Mexico. Comp Parasitol. 2001; 68:196–203. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=1145995
- Salgado-Maldonado G. Zootaxa: Checklist of helminth parasites of freshwater fishes from Mexico. Zootaxa. 2006; 1324(1):1-357. https://doi.org/10.11646/zootaxa.1324.1.1
- Violante-González J, Aguirre-Macedo ML. Metazoan parasites of fishes from Coyuca Lagoon, Guerrero, Mexico. Zootaxa. 2007; 1531:39–48. http://recursosnaturales.uagro.mx/Docs/Articulos%20PDF/025%20Violante%20y%20Aguirre%20(2007).pdf
- Lira-Guerrero G, García-Prieto L, Pérez-Ponce de León G. Helminth parasites of atherinopsid freshwater fishes (Osteichthyes : Atheriniformes) from central Mexico. Rev Mex Biodivers. 2008; 79:325–31. http://www.ibiologia.unam.mx/barra/publicaciones/revista79_2/4-Lira%20et-192.pdf
- Violante-González J, Aguirre-Macedo ML, Vidal-Martínez VM. Temporal variation in the helminth parasite communities of the Pacific fat sleeper, Dormitator latifrons, from Tres Palos Lagoon, Guerrero, Mexico. J Parasitol. 2008; 94(2):326–334. https://doi.org/10.1645/GE-1251.1
- Alves-Pinto H, Lane de Melo A. Metacestodes of Parvitaenia macropeos (Cyclophyllidea, Gryporhynchidae) in Australoheros facetus (Pisces, Cichlidae) in Brazil. Neotrop Helminthol. 2011; 5(2):279–283. https://dialnet.unirioja.es/servlet/articulo?codigo=3890246
- Alves-Pinto H, Lane de Melo A. Metacestodes of Glossocercus auritus (Cyclophyllidea, Gryporhynchidae) in Poecilia reticulata (Pisces, Poeciliidae) from Brazil. Rev Bras Parasitol Veterinária. 2011; 20(2):161–164. https://doi.org/10.1590/S1984-29612011000200012
- Bellay S, Hideo-Ueda B, Takemoto RM, Perez-Lizama M de los A, Pavanelli CG. Fauna parasitária de Geophagus brasiliensis (Perciformes: Cichlidae) em reservatórios do estado do Paraná, Brasil. Rev Bras Biociências. 2012; 10(1):74–78. https://www.seer.ufrgs.br/index.php/rbrasbioci/article/view/115605
- Monteiro CM, Santos MD, Zuchi NA, Brasil-Sato MC. Ecological parameters of the endohelminths in relation to size and sex of Prochilodus argenteus (Actinopterygii: Prochilodontidae) from the Upper São Francisco River, Minas Gerais, Brazil. Zoologia. 2009; 26(4):753–757. https://doi.org/10.1590/S1984-46702009000400021
- Bauer ON, Egusa S, Hoffman GL. Parasitic infections of economic importance in fishes. In W. Slusarski (Ed.). DWN Polish Scientific Publishers. Rev Adv Parasitol. 1981; 425–443. https://pubs.er.usgs.gov/publication/93994
- Saraiva A, Boane C, Cruz C. Effects of gryporhynchid metacestodes (Cestoda: Cyclophyllidea) in common carp (Cyprinus carpio) from Mozambique. Bull Eur Assoc Fish Pathol. 2009; 29(4):139–143. https://eafp.org/download/2009-Volume29/Issue%204/EAFP%20Bulletin%20294-5.pdf
- Morsy K, Bashtar A-R, Abdel-Ghaffar F, Al Quraishy S, Al Ghamdi A, Mostafa N. First identification of four trypanorhynchid cestodes: Callitetrarhynchus speciouses, Pseudogrillotia sp. (Lacistorhynchidae), Kotorella pronosoma and Nybelinia bisulcata (Tentaculariidae) from Sparidae and Mullidae fish. Parasitol Int. 2013; 112:2523–2532. https://doi.org/10.1007/s00436-013-3419-y
- Morsy K, Dajem S Bin, Al-kahtani M, El-kott A, Ibrahim E, Hamdi H, et al. Larval cestodes infecting commercial fish of Alexandria coast along the Mediterranean Sea: morphology and phylogeny. Rev Bras Parasitol Veterinária. 2022; 31(2):1–14. https://doi.org/10.1590/S1984-29612022030
- Santoyo-Telles F, Mariscal-Romero J, Gómez-Galindo C, Gutiérrez-Pulido H. Relaciones talla-peso y factor de condición de la tilapia Orecochromis niloticus en cinco cuerpos de agua del estado de Jalisco, México. Rev Iberoam las Ciencias Biológicas y Agropecu. 2019; 10(19). https://doi.org/10.23913/ciba.v8i16.92
- Leary S, Pharmaceuticals F, Ridge H, Underwood W, Anthony R, Cartner S, et al. AVMA Guidelines for the Euthanasia of Animals : 2020 Edition*. 2020. https://www.spandidos-publications.com/var/AVMA_euthanasia_guidelines_2020.pdf
- Scholz T, Kuchta R, Oros M. Tapeworms as pathogens of fish: A review. J Fish Dis. 2021; 44(12):1883–1900. https://doi.org/10.1111/jfd.13526
- Bush AO, Lafferty KD, Lotz JM, Shostak AW. Parasitology Meets Ecology on Its Own Terms : Margolis et al. Revisited. J Parasitol. 1997; 83(4):575–583. https://doi.org/10.2307/3284227
- Reiczigel J, Marozzi M, Fábián I, Rózsa L. Biostatistics for Parasitologists - A Primer to Quantitative Parasitology. Trends Parasitol [Internet]. 2019;35(4):277–281. Available from: https://doi.org/10.1016/j.pt.2019.01.003
- Scholz T, Bray RA, Kuchta R, Řepová R. Larvae of gryporhynchid cestodes (Cyclophyllidea) from fish : a review. Folia Parasitol (Praha). 2004; 51:131–152. https://doi.org/10.14411/fp.2004.018
- Bona FV. Family Dilepididae in Khalil et al. Keys the cestode parasites.pdf. In: Keys to the Cestode Parasites of Vertebrates. 1994. p. 443–554. https://www.cabdirect.org/cabdirect/abstract/19950800518
- Steckert LD, Cardoso L, Jerônimo GT, Benites de Pádua S, Martins ML. Investigation of farmed Nile tilapia health through histopathology. Aquaculture. 2018; 486:161–169. https://doi.org/10.1016/j.aquaculture.2017.12.021
- Hothorn T, Hornik K, Van de Wiel M, Zeileis A. A lego system for conditional inference. Am Stat. 2006; 60(3):257–263. https://doi.org/10.1198/000313006X118430.
- Ortega-Olivares MP, Barrera-Guzmán AO, Haasová I, Salgado-Maldonado G, Guillén-Hernández S, Scholz T. Tapeworms (Cestoda: Gryporhynchidae) of fish-eating birds (Ciconiiformes) from Mexico: New host and geographical records. Comp Parasitol. 2008; 75(2):182–195. https://doi.org/10.1654/4346.1
- Scholz T, Kuchta R, Salgado-Maldonado G. Cestodes of the family Dilepididae (Cestoda: Cyclophyllidea) from fish-eating birds in Mexico: a survey of species. Syst Parasitol. 2002; 52(3):171–182. https://doi.org/10.1023/a:1015700801579
- Scholz T, Boane C, Saraiva A. New Metacestodes of Gryporhynchid Tapeworms (Cestoda: Cyclophyllidea) from Carp (Cyprinus carpio Linnaeus, 1758) from Mozambique, Africa. Comp Parasitol. 2008; 75(2):315–320. http://www.bioone.org/doi/full/10.1654/4331.1
- Poulin R. Variation in the intraspecific relationship between fish length and intensity of parasitic infection: biological and statistical causes. J Fish Biol. 2000; 56:123–137. https://doi.org/10.1111/j.1095-8649.2000.tb02090.x
- Jarecka L. Life cycle of Valipora campylancristrota (Wedl, 1855) Baer and Bona 1958–1960 (Cestoda - Dilepididae) and the description of cercoscolex - a new type of cestode larva. Bull Polish Acad Sci. 1970; 28:99–102. https://www.cabdirect.org/cabdirect/abstract/19710801404
- Jarecka L. On the life cycles of Paradilepis scolecina (Rud., 1819) Hsü, 1935, and Neogryporhynchus cheilancristrotus (Wedl, 1855) Baer and Bona, 1958–1960 (Cestoda - Dilepididae). Bull I´Academie Pol des Sci Cl II Ser des Sci Biol. 1970; 18(3):159–163. https://www.cabdirect.org/cabdirect/abstract/19710801405
- Sysolyatina-Andakulova NA. The life cycle of the cestode Dilepis unilateralis. Parazity i Bolezn ryb, Moscow. 1979; 23:135–48.
- Ortiz-Galarza FM, Garzón-Santomaro C. New nesting locality of the Agami Heron, Agamia agami (Pelecaniformes: Ardeidae) in Ecuador. Biota Colomb. 2019; 20(1):126–131. https://doi.org/10.21068/c2019.v20n01a09
- Carrasco L. Nuevo registro de la Garza Cucharón Cochlearius cochlearius (Pelecaniformes: Ardeidae) en el occidente de Ecuador. Rev Ecuatoriana Ornitol. 2019; 5:14–18. https://doi.org/10.18272/reo.v0i5.1241
- Ordóñez-Delgado L, González I, Armijos-Ojeda D, Orihuela-Torres A. Primer registro de Ardea cocoi (Pelecaniformes: Ardeidae) en la región Andina del sur de Ecuador. Cedamaz. 2017; 7(1):10–15. https://revistas.unl.edu.ec/index.php/cedamaz/article/view/368/323
- Bahamonde-Vinueza D, Cadena-Ortiz H, Cajas-Bermeo C, Bonaccorso E. Unusual records of Cochlearius cochlearius (Linnaeus, 1766) (Aves: Ardeidae) in the Andes of Ecuador. Check List. 2014; 10(3):687–688. https://doi.org/10.15560/10.3.687
- Laboni NN, Chandra KJ, Chhanda MS. Effects of Caryophyllaeid cestode infestations on Clarias batrachus (Linn.). J Asiat Soc Bangladesh, Sci. 2012; 38(2):135–144. https://doi.org/10.3329/jasbs.v38i2.15594
- Scholz T, Davidovich N, Aflalo O, Hadar S, Mazuz ML, Yasur-Landau D. Invasive Amirthalingamia macracantha (Cestoda: Cyclophyllidea) larvae infecting tilapia hybrids in Israel: a potential threat for aquaculture. Dis Aquat Organ. 2021; 145:185–190. http://dx.doi.org/10.3354/dao03611
- Delgado-Delgado DD, Morán-Caicedo IA, Holguín-Burgos B. Producción y exportación del chame en el Ecuador en el período 2013 - 2016. Obs la Econ Latinoam. 2018; 1–13. https://www.eumed.net/rev/oel/2018/09/produccion-chame-ecuador.html
- OIE. Medidas Zoosanitarias que se deben aplicar antes de la salida y a la salida. In: (OIE) OM de la SA, editor. Código Sanitario para los Animales Acuáticos [Internet]. 2021. p. 1–2. https://www.woah.org/es/que-hacemos/normas/codigos-y-manuales/acceso-en-linea-al-codigo-acuatico/?id=169&L=1&htmfile=chapitre_aahm_before_and_at_departure.htm