Histopathological findings in Anisakidae nematodes exposed to aqueous plant extracts with nematicidal capacity in vitro
Hallazgos histopatológicos en nematodos Anisakidae, expuestos a extractos acuosos vegetales con capacidad nematicida in vitro

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
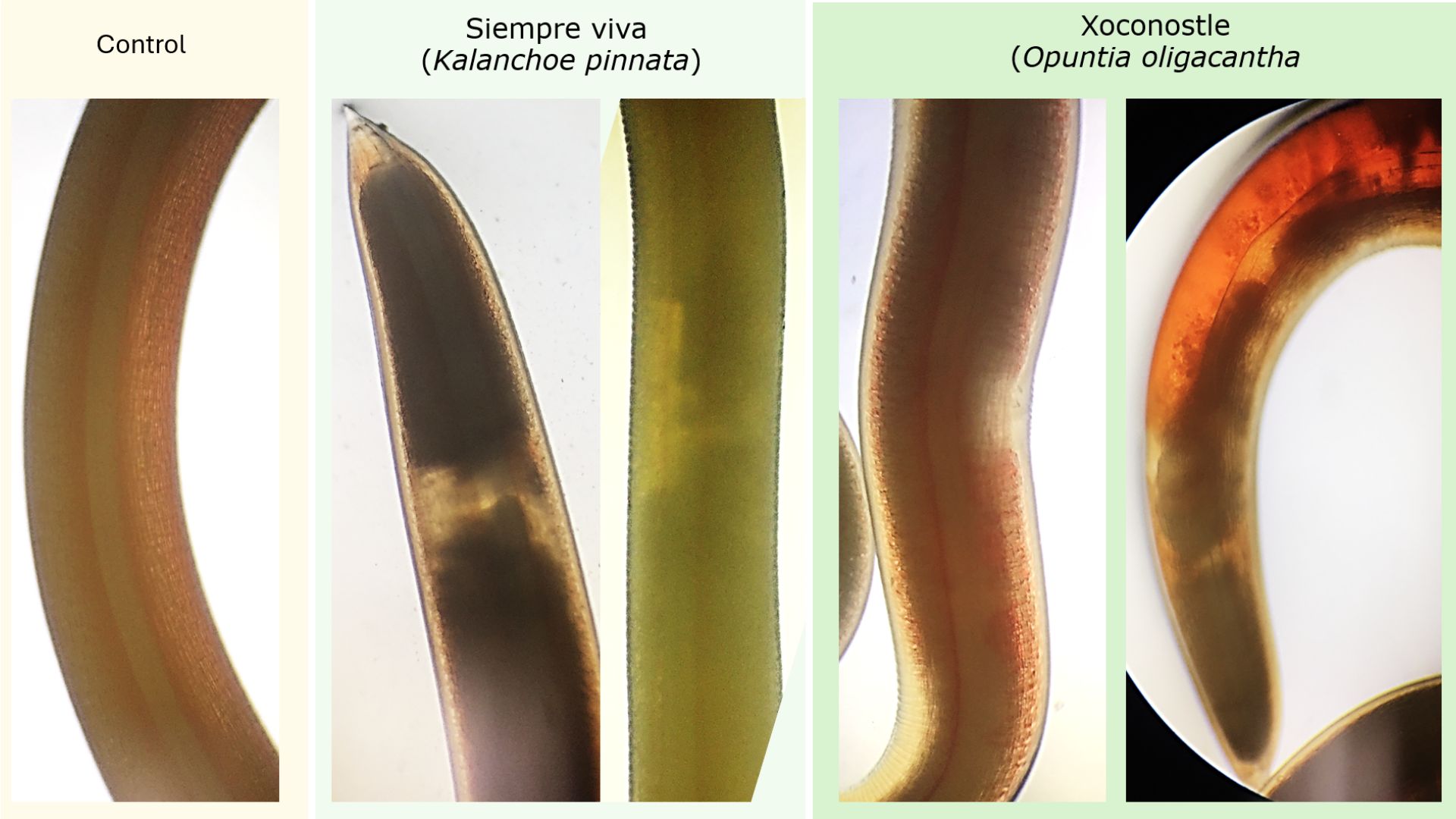
Objetive. Expose nematodes of the Anisakidae family to different aqueous extracts and identify the aqueous extracts with nematicidal capacity. Material and methods. The concentrations of the bioactive compounds of the aqueous extracts of epazote (Dysphania ambrosioides), onion (Allium cepa), siempre viva (Kalanchoe pinnata) and xoconostle (Opuntia oligacantha) were identified. Live parasites of the Anisakidae family were obtained from Lisa fish (Mugilidae), to be exposed to different concentrations of aqueous extracts. Results. K. pinnata and O. oligocantha presented a higher concentration of bioactive components of polyphenols, flavonoids and tannins; as well as in the antioxidant activity of DPPH and ABTS. Parasite mortality occurred at a concentration of 855 mg/mL for K. pinnata of 100% at 48 hours, and in pulp and whole fruit of O. oligacantha, with mortality of 66% at 72 hours. The main histopathological changes caused by K. pinnata were muscle vacuoles; the whole fruit of O. oligocantha degeneration of the intestinal epithelium and vacuolization; the seed caused edema, intestinal degeneration, and vacuolization. Conclusions. The results indicate that the use of aqueous extracts of K. pinnata and O. oligacantha on nematodes of the Anisakidae family are an option for their use as nematicidal agents.
Article visits 395 | PDF visits
Downloads
- Ángeles-Hernández JC, Gómez-de Anda FR, Reyes-Rodríguez NE, Vega-Sánchez V, García-Reyna PB, Campos-Montiel RG, et al. Genera and species of the Anisakidae family and their geographical distribution. Animals (Basel). 2020; 10:2374. https://doi.org/10.3390/ani10122374
- Pekmezci GZ. Occurrence of Anisakis pegreffii (Nematoda: Anisakidae) Larvae in Imported John Dory (Zeus faber) from Senegalese Coast Sold in Turkish Supermarkets. Acta Parasitol. 2019; 64:582–586. https://doi.org/10.2478/s11686-019-00078-0
- Castellanos JA, Tangua AR, Salazar L. Anisakidae nematodes isolated from the flathead grey mulletfish (Mugilcephalus) of Buenaventura, Colombia. IJP-PAW. 2017; 6:265–270. https://doi.org/10.1016/j.ijppaw.2017.08.001
- Tokiwa T, Kobayashi Y, Ike K, Morishima Y, Sugiyama H. Detection of Anisakid Larvae in Marinated Mackerel Sushi in Tokyo, Japan. Jpn J Infect Dis. 2018; 71:88–89. https://doi.org/10.7883/yoken.JJID.2017.280
- Amir A, Ngui R, Ismail WH, Wong KT, Ong JS, Lim YA, Lau YL, Mahmud R. Anisakiasis Causing Acute Dysentery in Malaysia. Am. J. Trop. Med. Hyg. 2016; 95:410–412. https://doi.org/10.4269/ajtmh.16-0007
- Nieuwenhuizen NE, Lopata AL. Allergic reactions to Anisakis found in fish. Curr Allergy Asthma Rep. 2014; 14:455. https://doi.org/10.1007/s11882-014-0455-3
- Cabrera-Carrión JL, Jaramillo-Jaramillo C, Dután-Torres F, Cun-Carrión J, García PA, Astudillo LR. Variación del contenido de alcaloides, fenoles, flavonoides y taninos en Moringa oleifera Lam. en función de su edad y altura. Bioagro. 2017; 29(1):53-60. https://www.redalyc.org/articulo.oa?id=85750098006
- Bernal-Peralta A, Camargo-Silva A. Efecto in vitro de los taninos condensados de las plantas Leucaena Leucocephala, Calliandra calothyrsus y Flemingia macrophylla sobre huevos y larvas (L3) de nematodos gastrointestinales de ovinos [Master Tesis]. Bogotá, Colombia: Universidad de la Salle; 2016.
- Barros L, Pereira E, Calhelha RC, Dueñas M, Carvalho AM, Santos-Buelga C, Ferreira ICFR. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. JFF. 2013; 5(4):1732-1740. https://doi.org/10.1016/j.jff.2013.07.019
- Orengo K, Maitho T, Mbaria JNM, Kitaa J. In vitro anthelmintic activity of Allium sativum, Allium cepa and Jatropha curcas against Toxocara canis and Ancylostoma caninum. Afr. J. Pharm Pharmacol. 2016; 10:465-471. https://doi.org/10.5897/AJPP2016.4551
- Alves CV, Santiago SRS da S, Soares ER, Almeida RA de, Lima BR de, Carvalho CSM de, Santiago PAL. Determination of the chemical profile extracts obtained from Kalanchoe pinnata (Lam.) Pers native of municipality Tabatinga-AM. RSD. 2022; 11(4):e1411427103. https://rsdjournal.org/index.php/rsd/article/view/27103
- Trujillo W, Betancourt VHG. Plantas medicinales utilizadas por tres comunidades indígenas en el noroccidente de la Amazonía (Colombia). Mundo Amazon. 2011; 2:283-306. https://revistas.unal.edu.co/index.php/imanimundo/article/view/14110
- Reyes-Rodríguez NE, Vega-Sánchez V, Gómez-de-Anda FB, García-Reyna PB, González R, Zepeda-Velázquez AP. Species of Anisakidae nematodes and Clinostomum spp. Infecting lisa Mugil curema (Mugilidae) intended for human consumption in Mexico. Rev Bras Parasitol Vet. 2020; 29 (1):e017819. https://doi.org/10.1590/S1984-29612020002
- Noga EJ. Fish disease, diagnosis, and treatment. 2nd ed. USA: Wiley-Blackwell; 2010.
- López V, Cascella M, Benelli G, Maggi F, Gómez-Rincón C. Green drugs in the fight against Anisakis simplex—larvicidal activity and acetylcholinesterase inhibition of Origanum compactum essential oil. Parasitol Res. 2018; 117(3):861-867. https://doi.org/10.1007/s00436-018-5764-3
- Medina-Pérez G, Zaldívar-Ortega AK, Cenobio-Galindo ADJ, Afanador-Barajas LN, Vieyra-Alberto R, Estefes-Duarte JA, Campos-Montiel RG. Antidiabetic Activity of Cactus Acid Fruit Extracts: Simulated Intestinal Conditions of the Inhibitory Effects on α-amylase and α-glucosidase. Applied Sciences. 2019; 9(19):4066. https://doi.org/10.3390/app9194066
- Soto M, Rosales M. Efecto del solvente y de la relación masa/solvente, sobre la extracción de compuestos fenólicos y la capacidad antioxidante de extractos de corteza de Pinus durangensis y Quercus sideroxyla. Maderas, Cienc Tecnol. 2016; 18(4):701-714. http://dx.doi.org/10.4067/S0718-221X2016005000061
- Singlenton VL, Rudolf O, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folinciocalteu reagent. Methods Enzymol. 1999; 299:152-178. https://doi.org/10.1016/S0076-6879(99)99017-1
- Tommasi F, Paciolla C, Arrigoni O. The ascorbate system in recalcitrant and orthodox seeds. Physiol Plant. 1999; 105(2):193-198. https://doi.org/10.1034/j.1399-3054.1999.105202.x
- Price ML, Larry GB. Rapid visual estimation and spectrophotometric determination of tannin content of sorghum grain. J Agric Food Chem. 1977; 25:1268-1273. https://doi.org/10.1021/jf60214a034
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Food Sci Technol. 1995; 28(1):25-30. https://doi.org/10.1016/S0023-6438(95)80008-5
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999; 26(9-10):1231-1237. https://doi.org/10.1016/S0891-5849(98)00315-3
- Giarratana F, Muscolino D, Ziino G, Lo Presti V, Rao R, Chiofalo V, Panebianco A. Activity of Catmint (Nepeta cataria) essential oil against Anisakis spp larvae. Tropical Biomed. 2017; 34(1):22-31. https://www.msptm.org/files/Vol34No1/022-031-Giarratana-F.pdf
- Zepeda-Velázquez AP, Vega-Sánchez V, Ortega-Santana C, Rubio-Godoy M, de Oca-Mira DM, Soriano-Vargas E. Pathogenicity of Mexican isolates of Aeromonas sp. in immersion experimentally- infected rainbow trout (Oncorhynchus mykiss, Walbaum 1792). Acta Tropica. 2017; 169: 22–124. https://doi.org/10.1016/j.actatropica.2017.02.013
- Gómez-Rincón C, Langa E, Murillo P, Valero MS, Berzosa C, López V. Activity of tea tree (Malelulca alternifolia) essential oil against L3 larvae of Anisakis simplex. Biomed Res Int. 2014; 2014:549510. https://doi.org/10.1155/2014/549510
- Suzuki, T. y Yamato, S. Mode of action of piperovatine, an insecticidal piperamide isolated from Piper piscatorum (Piperaceae), against voltage-gated sodium channels Neurotoxicology. 2018; 69:288-295. https://doi.org/10.1016/j.neuro.2018.07.021
- Castro-Parra L, Reyes-Munguía A, Aguirre-Álvarez G, Zepeda-Velázquez AP, Mejía-Islas AK, Almaraz-Buendía I, Espino-García JJ, Campos-Montiel RG. Efecto de los extractos acuosos de Dysphania ambrosioides (epazote) y Allium cepa (cebolla) sobre nematodos de la familia Anisakidae encontrados en peces de Mugil curema (Lisa). Academia Journals. 2020; 12(7):347-351. https://static1.squarespace.com/static/55564587e4b0d1d3fb1eda6b/t/5f935efc1d206d227a5067ea/1603493649132/Tomo+03+-+Memorias+del+Congreso+Internacional+AJ+Hidalgo+2020.pdf
- Fernández-Luqueño F, Medina-Pérez G, Pérez-Soto E, Espino-Manzano S, Peralta-Adauto L, Pérez-Ríos S, Campos-Montiel R. Bioactive Compounds of Opuntia spp. Acid Fruits: Micro and Nano-Emulsified Extracts and Applications in Nutraceutical Foods. Molecules. 2021; 26(21):6429. https://doi.org/10.3390/molecules26216429
- Báez M, Torres EI, Gruszycki AE, Alba DA, Valenzuela GM, Gruszycki MR. Actividad antioxidante y antiinflamatoria en extractos hidroalcohólicos de Kalanchoe daigremontiana Raym. -Hamet & H. Perrier. Rev Colomb Cienc Quim Farm. 2021; 50(1):86-99. https://doi.org/10.15446/rcciquifa.v50n1.95450.
- Hernández-Fuentes AD, Trapala-Islas A, Gallegos-Vásquez C, Campos-Montiel RG, Pinedo-Espinoza JM, Guzmán-Maldonado SH. Physicochemical variability and nutritional and functional characteristics of xoconostles (Opuntia spp.) accessions from Mexico. Fruits. 2015; 70:109-116. https://doi.org/10.1051/fruits/2015002.
- Bogucka-Kocka A, Zidorn C, Kasprzycka M, Szymczak M, Szewczyk K. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi J Biol Sci. 2018; 25(4):622-630. https://doi.org/10.1016/j.sjbs.2016.01.037
- Cenobio-Galindo AJ, Díaz-Monroy G, Medina-Pérez G, Franco-Fernández MJ, Ludeña-Urquizo FE, Vieyra-Alberto R, Campos-Montiel RG. Multiple Emulsions with Extracts of Cactus Pear Added in A Yogurt: Antioxidant Activity, In Vitro Simulated Digestion and Shelf Life. Foods. 2019; 8(10):429. https://doi.org/10.3390/foods8100429
- Kolodziejczyk-Czepas J. Stochmal A. Bufadienolides of Kalanchoe species: an overview of chemical structure, biological activity and prospects for pharmacological use Phytochem Rev. 2017; 16(6):1155-1171. https://doi.org/10.1007/s11101-017-9525-1
- Medina-Pérez G, Peralta-Adauto L, Afanador-Barajas L, Fernández-Luqueño F, Pérez-Soto E, Campos-Montiel R, Peláez-Acero A. Inhibition of Urease, Elastase, and β-Glucuronidase Enzymatic Activity by Applying Aqueous Extracts of Opuntia oligacantha CF Först Acid Fruits: In Vitro Essay under Simulated Digestive Conditions. Appl Sci. 2021; 11:7705. https://doi.org/10.3390/app11167705
- Sekeroglu N, Senol FS, Orhan IE, Gulpinar AR, Kartal M,Sener B. In vitro prospective effects of various traditional herbal coffees consumed in Anatolia linked to neurodegeneration. Int Food Res J. 2012; 45:197-203. https://doi.org/10.1016/j.foodres.2011.10.008
- Osorio-Esquivel O, Ortiz-Moreno A, Garduño-Siciliano L, Alvarez VB, Hernández-Navarro MD. Antihyperlipidemic effect of methanolic extract from Opuntia joconostle seeds in mice fed a hypercholesterolemic diet. Plant Foods Hum Nutr. 2012; 67(4):365-70. https://doi.org/10.1007/s11130-012-0320-2
- Barros LA, Yamanaa AR, Silva LE, Vaneler MLA, Braum DT, Bonaldo J. In vitro larvacidal activity of geraniol and citronellal against Contracaecum sp (Nematoda: Anisakidae). Braz J Med Biol Res. 2009; 42(10):918-20. https://doi.org/10.1590/S0100-879X2009005000023.
- Romero MC, Navarro MC, Martín-Sánchez J, Valero A. Peppermint (Mentha piperita) and albendazole against Anisakiasis in an animal model. Trop Med Int Health. 2014; 19(12):1430-6. https://doi.org/10.1111/tmi.12399
- Romero MC, Valero A, Martín-Sánchez J, Navarro-Moll MC. Activity of Matricaria chamomilla essential oil anisakiasis. Phy- Tomedicine. 2012; 19(6):520-523. https://doi.org/10.1016/j.phymed.2012.02.005.























