Microbiological quality and antibacterial activity of honey produced by Melipona beecheii in Yucatan, Mexico
Calidad microbiológica y actividad antibacteriana de miel producida por Melipona beecheii en Yucatán, México

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
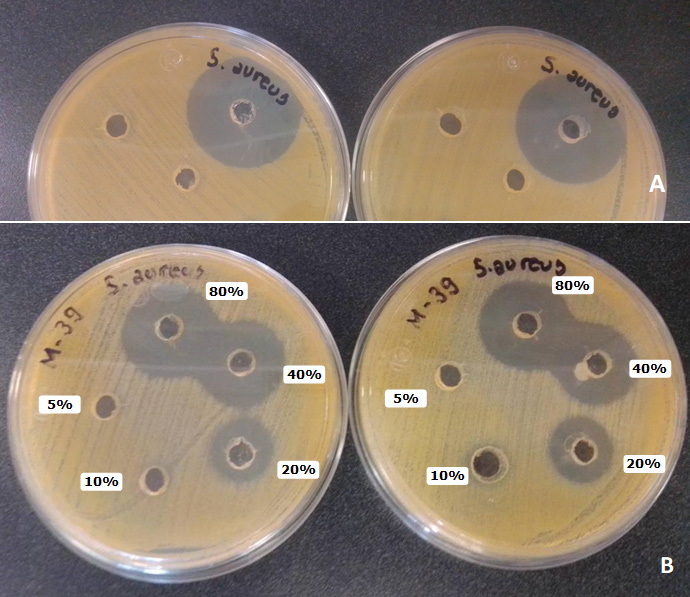
Objective. To analyze the microbiological quality and antibacterial activity in 43 samples of honey produced by Melipona beecheii, extracted during the years 2020 and 2021 in the harvest (January to May) and post-harvest (June to October) seasons from meliponaries located in the low deciduous forest of southeast of Mexico. Materials and methods. Microbiological quality was determined by evaluating the content of total aerobic mesophiles, coliforms, molds, yeasts, and spore-forming anaerobes. For antibacterial activity, the agar well diffusion assay was used using 5, 10, 20, 40 and 80% honey concentrations. Results. The presence of aerobic mesophiles (83.7% of the samples), coliforms (4.6%), molds (20.9%) and yeasts (39.5%) was observed, with a maximum of 4.5x102, 2.5x10, 9.5x10 and 3.2x103 CFU/g, respectively. The presence of sporulated forms of sulfite-reducing clostridia was not observed in any sample. With respect to the antibacterial activity, the highest zones of inhibition were recorded against Staphylococcus aureus at a honey concentration of 80 and 40%, contrary to what was observed with Salmonella var. Typhimurium, Pseudomonas aureginosa and Escherichia coli, where the interference in bacterial growth was not so evident. Conclusions. However, the growth of mesophiles and yeasts in most of the samples showed antibacterial activity against the pathogens mentioned above, which can be attributed to the interactions between microbiome, plants, bees and physicochemical characteristics of honey.
Article visits 532 | PDF visits
Downloads
- Bratman EZ. Saving the Other Bees: The Resurgence of Stingless Beekeeping in the Zona Maya. Conserv Soc. 2020; 18(4):387-398. https://doi.org/10.4103/cs.cs_20_66
- Marconi M, Ormeño-Luna J, Vecco-Giove CD. Physicochemical and microbiological quality of honeys produced by stingless bees Scaptotrigona polysticta, Melipona illota and Tetragonisca angustula (Apidae: Meliponini) in San Martín, Peru. Peruv J Agron. 2020; 4(2): 55-60. https://doi.org/10.21704/pja.v4i2.1541
- Pinheiro CG, Abrantes MR, Silva RO, Junior CA, Lobato FC, Silva JB. Microbiological quality of honey from stingless bee, jandaíra (Melipona subnitida), from the semiarid region of Brazil. Ciênc Rural. 2018; 48(9):e20180151. https://doi.org/10.1590/0103-8478cr20180151
- Ngalimat MS, Raja Abd Rahman RNZ, Yusof MT, Syahir A, Sabri S. Characterisation of bacteria isolated from the stingless bee, Heterotrigona itama, honey, bee bread and propolis. Peer J. 2019; 7:e7478. https://doi.org/10.7717/peerj.7478
- Vit P, Medina M, Enríquez ME. Quality standards for medicinal uses of Meliponinae honey in Guatemala, Mexico and Venezuela. Bee World. 2004; 85(1):2-5. https://doi.org/10.1080/0005772X.2004.11099603
- Rao PV, Krishnan KT, Salleh N, Gan SH. Biological and therapeutic effects of honey produced by honey bees and stingless bees: a comparative review. Rev Bras Farmacogn. 2016; 26(5):657-664. https://doi.org/10.1016/j.bjp.2016.01.012
- Domingos SCB, Clebis VH, Nakazato G, de Oliveira AGJr, Takayama Kobayashi RK, Peruquetti RC, et al. Antibacterial activity of honeys from Amazonian stingless bees of Melipona spp. and its effects on bacterial cell morphology. J Sci Food Agric. 2021; 101(5):2072-2077. https://doi.org/10.1002/jsfa.10828
- Brown E, O'Brien M, Georges K, Suepaul S. Physical characteristics and antimicrobial properties of Apis mellifera, Frieseomelitta nigra and Melipona favosa bee honeys from apiaries in Trinidad and Tobago. BMC Complement Altern Med. 2020; 20(1):85. https://doi.org/10.1186/s12906-020-2829-5
- Ng WJ, Sit NW, Ooi PA, Ee KY, Lim TM. The antibacterial potential of honeydew honey produced by stingless bee (Heterotrigona itama) against antibiotic resistant bacteria. Antibiotics (Basel). 2020; 9(12):871. https://doi.org/10.3390/antibiotics9120871.
- Albaridi NA. Antibacterial potency of honey. Int J Microbiol Res. 2019; 2: 2464507. https://doi.org/10.1155/2019/2464507.
- Brudzynski K. Honey as an ecological reservoir of antibacterial compounds produced by antagonistic microbial interactions in plant nectars, honey and honey bee. Antibiotics. 2021; 10(5):551. https://doi.org/10.3390/antibiotics10050551
- Almasaudi S. The antibacterial activities of honey. Saudi J Biol Sci. 2021; 28(4):2188-2196. https://doi.org/10.1016/j.sjbs.2020.10.017.
- Castillo CAV, Moguel OYB, Cortés CMA, Espinosa HA, Arechavaleta VME, Mora AMA. Composición botánica de mieles de la península de Yucatán, mediante qPCR y análisis de curvas de disociación. Rev Mex Cienc Pecu. 2016; 7(4):489–505. https://doi.org/10.22319/rmcp.v7i4.4278
- Zamudio AC. Producción de miel convencional y orgánica en la península de Yucatán. (Tesis de maestría). México: El Colegio de la Frontera Sur; 2017. https://ecosur.repositorioinstitucional.mx/jspui/handle/1017/1932
- Tanuğur-Samanc AE, Kekeçoğlu M. An evaluation of the chemical content and microbiological contamination of Anatolian bee venom. PLoS One. 2021; 16(7):e0255161. https://doi.org/10.1371/journal.pone.0255161
- Trinks F. Análisis microbiológico de los alimentos. Metodología analítica oficial. Microorganismos indicadores. Volumen 3. ReNaLOA-ANMAT: Argentina; 2014. http://www.anmat.gov.ar/renaloa/docs/analisis_microbiologico_de_los_alimentos_vol_iii.pdf
- Ramírez-Miranda I, Betancur-Ancona D, Moguel-Ordóñez Y. Physicochemical and microbiological standards of honey produced by genus Melipona. J Apic Sci. 2021; 65(2):197-216. https://doi.org/10.2478/jas-2021-0016
- Caldas MJM, Silva IP, Machado CS, Carvalho CAL, Sodré G da S. Qualidade e perfil antimicrobiano do mel de Melipona asilvai. Braz J Dev. 2020; 6(5):32760–32768. http://dx.doi.org/10.34117/bjdv6n5-646
- Fernandes RT, Rosa IG, Conti-Silva AC. Microbiological and physical-chemical characteristics of honeys from the bee Melipona fasciculata produced in two regions of Brazil. Ciência Rural. 2018; 48(5): e20180025. https://doi.org/10.1590/0103-8478cr20180025
- Nadja JW, Ajit A, Naila A, Sulaiman AZ, Naila A. Physicochemical and microbiological analysis of stingless bees honey collected from local market in Malaysia. Indones J Chem. 2019; 19(2):522-530. https://doi.org/10.22146/ijc.40869
- Vázquez-Quiñones CR, Moreno-Terrazas R, Natividad-Bonifacio I, Quiñones-Ramírez EI, Vázquez-Salinas C. Microbiological assessment of honey in México. Rev Argent Microbiol. 2018; 50(1):75-80. https://doi.org/10.1016/j.ram.2017.04.005.
- Schencke C, Vásquez B, Sandoval C, del Sol M. El rol de la miel en los procesos morfofisiológicos de reparación de heridas. Int. J. Morphol 2016; 34(1):385-395. https://dx.doi.org/10.4067/S0717-95022016000100056
- Abdulla CO, Ayubi A, Zulfiquer F, Santhanam G, Ahmed MA, Deeb J. Infant botulism following honey ingestion. BMJ Case Rep. 2012: bcr1120115153. https://doi.org/10.1136/bcr.11.2011.5153
- Silva MS, Rabadzhiev Y, Eller MR, Ilia Iliev I, Ivanova I, Santana WC. Microorganisms in Honey. In (Ed.), Honey Analysis. IntechOpen. 2017. http://dx.doi.org/10.5772/67262
- Beux MR, Ávila S, Surek M, Bordin K, Leobet J, Barbieri F et al. Microbial biodiversity in honey and pollen pots produced by Tetragonisca angustula (Jataí). Biol Appl Sci. 2022; 65:e22210440. https://doi.org/10.1590/1678-4324-2022210440
- Santos AC, Biluca FC, Braghini F, Gonzaga LV, Oliveira Costa AC, Fett R. Phenolic composition and biological activities of stingless bee honey: An overview based on its aglycone and glycoside compounds. Food Res Int. 2021; 147(2021):110553. https://doi.org/10.1016/j.foodres.2021.110553.
- Silhavy TJ, Kahne D, Walker S. The bacterial cell envelope. Cold Spring Harb Perspect Biol. 2010; 2(5):a000414. https://doi.org/10.1101/cshperspect.a000414.
- Chan-Rodríguez D, Ramón-Sierra J, Lope-Ayora J, Sauri-Duch E, Cuevas-Glory L, Ortiz-Vázquez E. Antibacterial properties of honey produced by Melipona beecheii and Apis mellifera against foodborn microorganisms. Food Sci Biotechnol. 2012; 21(3):905-909. https://doi.org/10.1007/s10068-012-0118-x
- Dória MM, Dória PE, Trovatti UAP, Lucchese AM. Antimicrobial activity of honey from five species of Brazilian stingless bees. Ciênc Rural. 2013; 43(4):672-675. https://doi.org/10.1590/S0103-84782013005000016
- Salminen JP, Roslin T, Karonen M, Sinkkonen J, Pihlaja K, Pulkkinen P. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J Chem Ecol. 2004; 30(9):1693-1711. https://doi.org/10.1023/b:joec.0000042396.40756.b7
- Bouarab-Chibane L, Forquet V, Lantéri P, Climent Y, Léonard-Akkari L, Oulahal N et al. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front Microbiol. 2019; 10:829. https://doi.org/10.3389/fmicb.2019.00829
- Ruiz-Ruiz JC, J. Matus-Basto AJ, Acereto-Escoffié P, Segura-Campos MR. Antioxidant and anti-inflammatory activities of phenolic compounds isolated from Melipona beecheii honey. Food Agric Immuno. 2017; 28:1424-1437. https://doi.org/10.1080/09540105.2017.1347148
- Alvarez SM, Giampieri F, Brenciani A, Mazzoni L, Gasparrini M, González AM, et al. Apis mellifera vs Melipona beecheii Cuban polifloral honeys: A comparison based on their physicochemical parameters, chemical composition and biological properties. LWT - Food Sci. Technol. 2018; 87:272-279. https://doi.org/10.1016/j.lwt.2017.08.079
- Lee H, Churey JJ, Worobo RW. Antimicrobial activity of bacterial isolates from different floral sources of honey. Int J Food Microbiol. 2008; 126(1-2):240-244. https://doi.org/10.1016/j.ijfoodmicro.2008.04.030
- Rosli FN, Hazemi MHF, Akbar MA, Basir S, Kassim H, Bunawan H. Stingless bee honey: Evaluating its antibacterial activity and bacterial diversity. Insects. 2020; 11(8):500. https://doi.org/10.3390/insects11080500























