Clinical protocol for the care of ophidiotoxicosis in canines in Colombia
Protocolo clínico para la atención de la ofidiotoxicosis en caninos en Colombia

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
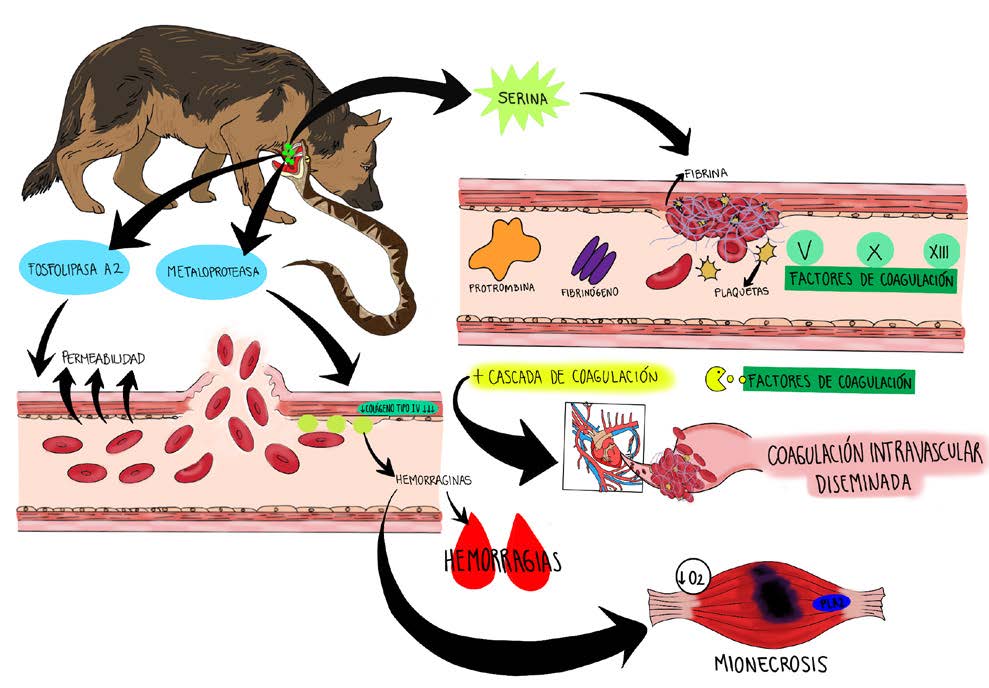
Every year, according to the World Health Organization, nearly 5,000,000 million accidents due to snake bites are reported worldwide, of which it is estimated that 33.3% to 50% of cases present as poisoning. Ophidic accidents that include ophidiotoxicosis have care protocols of therapy with antivenom serums, which vary according to the genus and species of the snake. Although in Colombia there are protocols for the care of ophidiotoxicosis in humans described by health entities, the canine clinic lacks this information. The aim of this systematic review is to build a medical protocol for ophidiotoxicosis in canines based on information reported in Colombia or tropical countries with which similar venomous snakes are shared. For this action, the PRISMA protocol was used; in total, 57 articles and 10 official documents on protocols for the care of ophidic accidents in humans were reviewed, which allowed the possible to classify snakebite accidents in group 1 (Bothrops, Lachesis and Crotalus accidents) and group 2 (Micrurus accident), also establish a medical protocol for ophidiotoxicosis in each of the groups indicated in canines, depending on the severity of the clinical and paraclinical condition. In conclusion, the clinical and paraclinical signs of the canine, vasculotoxic or neurotoxic, allow us to identify the group to which the aggressor snake belongs (group 1 or group 2, respectively), and their severity guides the selection and dosage of antivenom therapy specific for the clinical management of ophidiotoxicosis in canines.
Article visits 258 | PDF visits
Downloads
- Urbina J. Gradientes andinos en la diversidad y patrones de endemismo en anfibios y reptiles de Colombia: Posibles respuestas al cambio climático. Bistua. 2011; 7(1):74-91. https://doi.org/10.18359/rfcb.2065
- Cubillos S, Alarcón J. Accidente ofídico en Antioquia, Colombia: análisis etnobiológico de las construcciones culturales. Rev Etnobio. 2018; 16(2):18-29. https://revistaetnobiologia.mx/index.php/etno/article/view/304/303
- da Graça M, de Oliveira P, Machado C. Epidemiologia dos acidentes por animais peçonhentos e a distribuição de soros: estado de arte e a situação mundial. Rev Salud Pública. 2018; 20(4):523-529. https://doi.org/10.15446/rsap.V20n4.70432
- Minghui R, Malecela M, Cooke E, Abela-Ridder B. WHO’s Snakebite Envenoming Strategy for prevention and control. Lancet Glob Health. 2019; 7:e837-e838. http://dx.doi.org/10.1016/
- Gutiérrez J, Calvete J, Habib A, Harrison R, Williams D, Warrell D. Snakebite envenoming. Nat Rev Dis Primers. 2017; 3:317079 https://doi.org/10.1038/nrdp.2017.63
- León-Núñez L, Camero-Ramos G, Gutiérrez J. Epidemiology of snakebites in Colombia (2008-2016). Rev Salud Pública. 2020. 22(3): 280–287. https://doi.org/10.15446/rsap.v22n3.87005
- Estrada-Gómez S, Vargas-Muñoz LJ, Higuita-Gutiérrez LF. Epidemiology of Snake Bites Linked with the Antivenoms Production in Colombia 2008-2020: Produced Vials Do Not Meet the Needs. Drug Healthc Patient Saf. 2022; 29(14):171-184. https://doi.org/10.2147/DHPS.S367757
- Otero R, Nuñez V, Osorio R, Gutierrez J, Giraldo C, Posada L. Ability of six Latin American antivenoms to neutralize the venom of mapanaequis (Bothrops atrox) from Antioquia and Chocó (Colombia). Toxicon. 1995; 33(6):809-815. https://doi.org/10.1016/0041-0101(95)00009-B
- Albuquerque P, Jacinto C, Silva G, Lima J, Veras M, Daher E. Acute kidney injury caused by Crotalus and Bothrops snake venom: a review of epidemiology, clinical manifestations and treatment. Rev Inst Med Trop. 2013; 55(5):295-301. https://doi.org/10.1590/S0036-46652013000500001
- Sutton N, Bates N, Campbell A. Canine adder bites in the UK: a retrospective study of cases reported to the Veterinary Poisons Information Service. Vet Rec. 2011; 169(23):607. https://doi.org/10.1136/vr.d4695
- Gilliam L, Brunker J. North American snake envenomation in the dog and cat. Vet Clin North Am. 2011; 41(6):1239-1259. https://doi.org/10.1016/j.cvsm.2011.08.008
- Martínez-Pérez A, Cruz-Quintero A, Agudelo-Vega A, Restrepo-Muñoz A, Estrada-Atehortúa A, Rodríguez-Vargas A et al. Guía para el manejo de Emergencias Toxicológicas. 2017; 499-507. Disponible en: https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/DE/GT/guias-manejo-emergencias-toxicologicas-outpout.pdf
- Otero R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon 2009; 54:998-1011. https://doi.org/10.1016/j.toxicon.2009.07.001
- Lervik J, Lilliehöök I, Frendin J. Clinical and biochemical changes in 53 Swedish dogs bitten by the European adder-Vipera berus. Acta Vet Scand. 2010; 52(1):26. https://doi.org/10.1186/1751-0147-52-26
- Page M, McKenzie J, Bossuyt P, Boutron I, Hoffmann TC, Mulrow C, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. https://doi.org/10.1136/bmj.n71
- Posada S. Aspectos epidemiológicos, clínicos y de tratamiento para el accidente ofídico en perros y gatos. Rev Medicina Vet. 2015; (30):151-167. https://doi.org/10.19052/mv.3619
- Maguiña C, Chincha O, Vilcapoma P, Morante D. Actualización en clínica y terapia de mordedura de serpiente (ofidismo). Rev Médica Hered. 2020; 31(1):48-55. http://dx.doi.org/10.19052/mv.3619
- Rafhaella C, Danilo L, Tássia R, Karina F, Marco A, Norival A et al. Cytotoxic and inflammatory potential of a phospholipase A2 from Bothrops jararaca snake venom. J Venom Anim Toxins Incl Trop Dis. 2018; 24:33 https://doi.org/10.1186/s40409-018-0170-y
- Camila R, Eric N, Wayne C, Juan R, Armando S, Rodrigo A et al. Neuromuscular activity of the venoms of the Colombian coral snakes Micrurus dissoleucus and Micrurus mipartitus: An evolutionary perspective. Toxicon. 2012; 59(1):132-142. https://doi.org/10.1016/j.toxicon.2011.10.017
- Nina C, Olazábal C, Quispe A, Alzamora S, Gomes H, Huancahuire V. Caracterización bioquímica del veneno de la serpiente Bothrops roedingeri Mertens 1942, y sus actividades edematógena, hemorrágica y miotóxica. Biomédica. 2020; 40(4):682-692. https://doi.org/10.7705%2Fbiomedica.5228
- Herrera C, Voisin M, Escalante T, Rucavado A, Nourshargh S, Gutiérrez J. Effects of PI and PIII snake venom haemorrhagic metalloproteinases on the microvasculature: A confocal microscopy study on the mouse cremaster muscle. PLoS One. 2016; 11(12):e0168643. https://doi.org/10.1371/journal.pone.0168643
- Shengwei X, Chunhong H. Synergistic strategies of predominant toxins in snake venoms. Toxicol Lett. 2018; 287:142-154. https://doi.org/10.1016/j.toxlet.2018.02.004
- Frare B, Silva Resende Y, Dornelas B, Jorge M, Souza V, Alves L, et al. Clinical, Laboratory, and Therapeutic Aspects of Crotalus durissus (South American Rattlesnake) Victims: A Literature Review. Biomed Res Int. 2019; 1345923. https://doi.org/10.1155%2F2019%2F1345923
- Duarte RCF, Rios DRA, Leite PM, Alves LC, Magalhães HPB, Carvalho MDG. Thrombin generation test for evaluating hemostatic effects of Brazilian snake venoms. Toxicon. 2019; 163:36-43. https://doi.org/10.1016/j.toxicon.2019.03.012
- Larréché S, Chippaux JP, Chevillard L, Mathé S, Résière D, Siguret V, Mégarbane B. Bleeding and Thrombosis: Insights into Pathophysiology of Bothrops Venom-Related Hemostasis Disorders. Int J Mol Sci. 2021; 22(17):9643. https://doi.org/10.3390/ijms22179643
- Sarkar S, Sinha R, Chaudhury AR, Maduwage K, Abeyagunawardena A, Bose N, et al. Snake bite associated with acute kidney injury. Pediatr Nephrol. 2021; 36(12):3829-3840. https://doi.org/10.1007/s00467-020-04911-x
- Hrovat A, Schoeman JP, de Laat B, Meyer E, Smets P, Goddard A, Nagel S, Daminet S. Evaluation of snake envenomation-induced renal dysfunction in dogs using early urinary biomarkers of nephrotoxicity. Vet J. 2013; 198(1):239-44. https://doi.org/10.1016/j.tvjl.2013.06.030
- Gunther M, Jaffey J, Evans J, Paige C. Case Report: Persistent Moderate-to-Severe Creatine Kinase Enzyme Activity Elevation in a Subclinical Dog. Front Vet Sci. 2021; 8:757294. https://doi.org/10.3389/fvets.2021.757294
- Castañeda FE, Echeverry DF, Buriticá EF. Manejo médico de un accidente ofídico en un perro causado por Bothrops asper: informe de caso. CES Medicina Vet Zootec. 2016; 11(1):100-109. http://dx.doi.org/10.21615/cesmvz.11.1.10
- Bolívar-Barbosa J, Rodríguez-Vargas A. Actividad neurotóxica del veneno de serpientes del género Micrurus y métodos para su análisis. Revisión de la literatura. Rev Fac Medicina. 2020; 68(3):453-462. https://doi.org/10.15446/revfacmed.v68n3.75992
- Kamiguti S, Cardoso JLC. Haemostatic changes caused by the venoms of South American snakes. Toxicon. 1989; 27(9):955-963. ISSN 0041-0101. https://doi.org/10.1016/0041-0101(89)90146-3
- Sevilla-Sánchez MJ, Ayerbe-González S, Bolaños-Bolaños E. Aspectos biomédicos y epidemiológicos del accidente ofídico en el departamento del Cauca, Colombia, 2009-2018. Biomedica. 2021; 41(2):314–337. https://doi.org/10.7705/biomedica.5853
- Feitosa ES, Sampaio V, Sachett J, Castro DB, Noronha M, Lozano JL, et al. Snakebites as a largely neglected problem in the Brazilian Amazon: Highlights of the epidemiological trends in the State of Amazonas. Rev Soc Bras Medicina Trop. 2015; 48(Suppl I):34-41. https://doi.org/10.1590/0037-8682-0105-2013
- Gómez JP, Gómez C, Gómez ML. Sueros antiofídicos en Colombia: análisis de la producción, abastecimiento y recomendaciones para el mejoramiento de la red de producción. Biosalud. 2017; 16(2):96-116. https://doi.org/10.17151/biosa.2017.16.2.9
- INVIMA. Dirección de medicamentos y productos biológicos alerta no. 292-2023. suero antiofidico polivalente liofilizado – suero antiofidico anticoral liofilizado. Laboratorios Probiol. Instituto Nacional de Vigilancia de Medicamentos y Alimentos: Colombia; 2023. https://app.invima.gov.co/alertas/ckfinder/userfiles/files/ALERTAS%20SANITARIAS/medicamentos_pbiologicos/2023/Septiembre/Alerta%20No_%20%23292-2023.pdf
- Russell JJ, Schoenbrunner A, Janis JE. Snake Bite Management: A Scoping Review of the Literature. Plast Reconstr Surg Glob Open. 2021; 9(4). https://doi.org/10.1097/GOX.0000000000003506
- Langston C, Gordon D. Effects of IV fluids in dogs and cats with kidney failure. Front Vet Sci. 2021; 8(659960):1-8. https://doi.org/10.3389/fvets.2021.659960
- Davis H, Jensen T, Johnson A, Knowles P, Meyer R, Rucinsky R, et al. AAHA/AAFP fluid therapy guidelines for dogs and cats. J Am Anim Hosp Assoc. 2013; 49(3):149-159. https://doi.org/10.5326/jaaha-ms-5868
- Bertola L, Cappelleri A, Tomba RM, Dotti E, Caniatti M, Dall’Ara P, et al. Vaccine-Associated Anaphylactic Shock in a Springer Spaniel Dog with Arrhythmogenic Right Ventricular Cardiomyopathy. J Comp Pathol. 2022; 194:34-38. https://doi.org/10.1016/j.jcpa.2022.03.009
- Shmuel DL, Cortes Y. Anaphylaxis in dogs and cats. J Vet Emerg Crit Care. 2013; 23(4):377-394. https://doi.org/10.1111/vec.12066
- Yadav SN, Ahmed N, Nath AJ, Mahanta D, Kalita MK. Urinalysis in dog and cat: A review. Vet World. 2020; 13(10):2133-2141. http://www.doi.org/10.14202/vetworld.2020.2133-2141
- Bustamante H, Werner M. Efecto sedativo de la asociación xilazina-morfina en caninos. Arch Med Vet. 2009; 41(3):229-236. http://dx.doi.org/10.4067/S0301-732X2009000300007