R. rattus and R. norvegicus, as reservoirs of zoonotic endoparasites in Ecuador
R. rattus y R. norvegicus, como reservorio de endoparásitos zoonóticos en Ecuador
Show authors biography
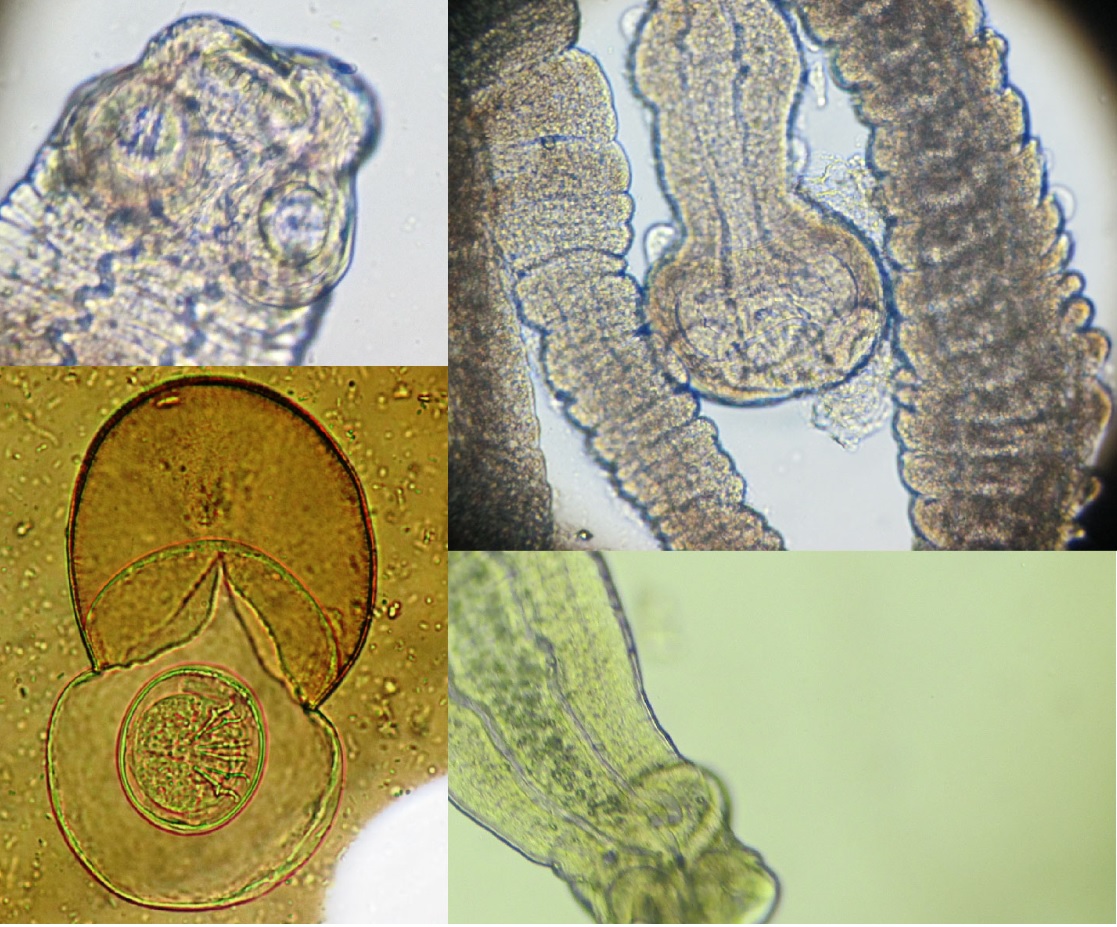
Objective. The present study was to identify gastro-intestinal endoparasitic helminths in wild rats. Materials and methods. a parasitological study was carried out to know the helminth-fauna in different urban and rural areas in five provinces of the Republic of Ecuador, during 2014-2017. The rodents were captured and transported to the National Parasitology Reference Center of the National Institute of Public Health Research - Guayaquil, for further analysis. Results. 125/211 (59.2%) rats with endoparasites were found, 13/20 (65%) for R. rattus and 112/191 (58.6%) R. norvegicus. The most prevalent nematode: was Nippostrongylus brasiliensis for both species and Heterakis spumosa; followed by the cestodes: Hymenolepis diminuta, H. nana, Moniliformis moniliformis and Cysticercus fasciolaris. Conclusions. The presence of zoonoses present in the rodents sampled that live near human communities, represent a potential risk of infection for the inhabitants. Therefore, the control of the population of rodents in residential areas and the awareness of the local population about the risk of disease transmission through rodents seems to be totally necessary.
Article visits 861 | PDF visits
Downloads
- UN-Habitat. Urbanization and Development: Emerging Features. United Nations Human Settlements Programme: Nairobi, Kenya; 2016. https://unhabitat.org/sites/default/files/download-manager-files/WCR-2016-WEB.pdf
- Carvalho-Pereira T, Souza F, Santos L, Walker R, Pertile A, Oliveira D, et al. The helminth community of a population of Rattus norvegicus from an urban Brazilian slum and the threat of zoonotic diseases. Parasitol. 2018; 145(6):797–806. http://dx.doi.org/10.1017/S0031182017001755
- Meerburg BG, Singleton GR, Kijlstra A. Rodent-borne diseases and their risks for public health. Crit Rev Microbiol.2009; 35:221–270. https://doi.org/10.1080/10408410902989837
- Luis AD, Hayman DT, O’Shea TJ et al. A comparison of bats and rodents as reservoirs of zoonotic viruses: Are bats special? Proc Biol Sci. 2013; 280:20122753. http://dx.doi.org/10.1098/rspb.2012.2753
- Chaisiri K, Siribat P, Ribas A, Morand S. Potentially zoonotic helminthiases of murid rodents fromthe Indo-Chinese peninsula: Impact of habitat and therisk of human infection. Vector-Borne Zoonotic Dises. 2015; 15:73–85. https://doi.org/10.1089/vbz.2014.1619
- Kosoy M, Khlyap L, Cosson J-F, Morand S. Aboriginal and invasive rats of genus Rattus as hosts ofinfectious agents. Vector-Borne Zoonotic Dises. 2015; 15:3–12. https://doi.org/10.1089/vbz.2014.1629
- Singleton GR, Belmain S, Brown PR, Aplin K, Htwe NM. Impacts of rodent outbreaks on food security in Asia. Wildlife Research. 2010; 37:355–359. https://doi.org/10.1071/WR10084
- John A. Rodent outbreaks and rice pre-harvestlosses in Southeast Asia. Food Security. 2014; 6:249–260. https://doi.org/10.1007/s12571-014-0338-4
- Wyatt KB, Campos PF, Gilbert MTP et al. Historical mammal extinction on Christmas island (Indian Ocean) correlates with introduced infectious disease. PLOS ONE. 2008; 3:e3602. https://doi.org/10.1371/journal.pone.0003602
- Morand S, Bordes F, Chen HW, Claude J, Cosson JF, et al. Global parasite and Rattus rodent invasions: The consequences for rodent-borne diseases. Integ Zoo. 2015; 10:409–423. http://dx.doi.org/10.1111/1749-4877.12143
- Kataranovski M, Mirkov I, Belij S, Popov A, Petrović Z, Gačić Z, Kataranovski D. Intestinal helminths infection of rats (Rattus norvegicus) In the Belgrade area (Serbia): the effect of sex, age and habitat. Parasite. 2011; 18:189-196. http://dx.doi.org/10.1051/parasite/2011182189
- Duque B, Aranzazu D, Agudelo-Flórez P, Londoño A, Quiroz V, Rodas J. Rattus norvegicus como indicador de la circulación de Capillaria hepatica y Taenia taeniaeformis en la Plaza Minorista de Medellín, Colombia. Biomédica. 2012; 32:510-518. http://dx.doi.org/10.7705/biomedica.v32i4.442
- Renzo de Sotomayor C, Serrano-Martínez E, Tantaleán M, Quispe HM, Casas VG. Identificación de Parásitos Gastrointestinales en Ratas de Lima Metropolitana. Rev Inv Pe. 2015; 26(2):273-281. https://doi.org/10.15381/rivep.v26i2.11003
- Martini RL, Dorta CA. Angiostrongylus cantonensis - Emergencia en América. 1th ed. Editorial Academia: La Habana; 2016. https://www.researchgate.net/publication/312031778_Angiostrongylus_cantonensis_Emergencia_en_America
- OPS. Manual para el control integral de Roedores. Organización Panamericana de la Salud: Colombia; 2012. https://www.minsalud.gov.co/sites/rid/Lists/BibliotecaDigital/RIDE/VS/PP/SA/manual-integral-de-roedores.pdf
- Ranjbar MJ, Sarkari B, Mowlavi GR, Seifollahi Z, Moshfe A, Abdolahi KS, Mobedi I. Helminth infections of rodents and their zoonotic importance in boyer-ahmad district, southwestern iran. Ira J Parasit. 2017; 12(4):572–579. https://ijpa.tums.ac.ir/index.php/ijpa/article/view/1931
- Abad AD, Chávez VA, Pinedo VR, Tantaleán VM, Gonzáles-Viera O. Helmintofauna Gastrointestinal de Importancia Zoonótica y sus Aspectos Patológicos en Roedores (Rattus spp) en Tres Medioambientes. Rev Invst Vet Pe. 2016; 27(4):736-750. http://dx.doi.org/10.15381/rivep.v27i4.12568
- Milazzo C, Cagnin M, Di BC, Geraci F, Ribas A. Helminth Fauna of Commensal Rodents, Mus musculus (Linnaeus, 1758) and Rattus rattus(Linnaeus, 1758) (Rodentia, Muridae) in Sicily (Italy). Rev Ibero-Latinoam Parasitol. 2010; 69(2):194-198.
- CCAC. Guide to the Care and Use of Experimental Animals. 2 Edition. Canadian Council Animal Care: Ottawa, Ontario; 2017. https://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf
- AMM. Declaración de la AMM sobre el Uso de Animales en la Investigación Biomédica. Asociación Médica Mundial; 2016. https://www.wma.net/es/policies-post/declaracion-de-la-amm-sobre-el-uso-de-animales-en-la-investigacion-biomedica/
- Anderson RC, Chabaud AG, Willmott S. Keys to the nematode parasites of vertebrates. Archivalvolume. Wallingford, UK: CAB International; 2009. https://www.cabi.org/bookshop/book/9781845935726/
- CDC. EpiInfo TM. Centers Disease Control; 2017. https://www.cdc.gov/epiinfo/index.html
- Franssen F, Swart A, Knapen F, Giessen J. Helminth parasites in black rats (Rattus rattus) and brown rats (Rattus norvegicus) from different environments in the Netherlands. Infect Ecol Epidemiol. 2016; 6:314-333. http://dx.doi.org/10.3402/iee.v6.31413
- Simões OR, Luque JL, Gentile R, Rosa MCS, Costa-Neto S, Maldonado A. Biotic and abiotic effects on the intestinal helminth community of the brown rat Rattus norvegicus from Rio de Janeiro, Brazil. J Helminthol. 2014; 90(1):21-27. http://dx.doi.org/10.3402/iee.v6.3141310.1017/S0022149X14000704
- Coomansingh C, Pinckney RD, Bahaiyat MI, Chikweto A, Bitner S, Baffa A, Sharma R. Prevalence of endoparasites wild rats in Grenada. St. George´s University. West Ind Vet J. 2009; 9(1):17-21. https://sta.uwi.edu/fms/vet/documents/3.pdf
- Akande AO. A study on wild rat behaviour and control on a pig farm. Master of Science Programme in Veterinary Medicine for International Students. Swedish: University of Agricultural Sciences; 2011. https://stud.epsilon.slu.se/3357/
- Gonçalves AL, Belizário TL, Pimentel JB, Penatti MP, Pedroso RS. Prevalence of intestinal parasites in preschool children in the region of Uberlândia, State of Minas Gerais, Brazil. Rev Soc Bras Med Trop. 2011; 44(2):191–193. https://doi.org/10.1590/S0037-86822011005000022
- Al-Salihi KA, Sheikh A, Saied H. Highly prevalence of Strobilocercus fasciolaris infection associated with gastroentropathy and hepatic fibrosarcoma between laboratory rats in experimental gropus. Afri J Anim Biomed Sci. 2009; 4(2):6-10. https://www.ajol.info/index.php/ajbr/issue/archive/2
- Rodríguez-Vivas R, Panti-May J, Parada-López J, Hernández-Betancourt S, Ruiz-Piña H. The occurrence of the larval cestode Cysticercus fasciolaris in rodent populations from the Cuxtal ecological reserve, Yucatan, Mexico. J Helminthol. 2011; 85(4):458-461. http://dx.doi.org/10.1017/S0022149X10000817























