Growth and survival of Cryphiops caementarius in coculture with Oreochromis niloticus at different densities
Crecimiento y supervivencia de Cryphiops caementarius en cocultivo con Oreochromis niloticus a diferentes densidades
Show authors biography
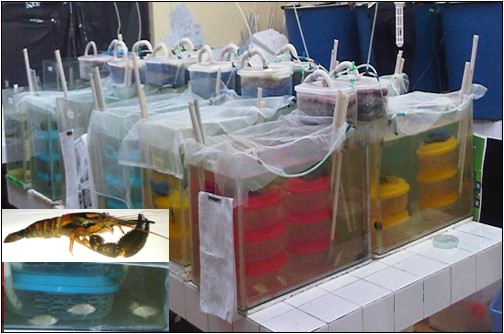
Objective. To evaluate the growth and survival of Cryphiops caementarius in coculture with Oreochromis niloticus at different densities. Materials and methods. Male prawns (5.86 cm and 7.65 g) and reverse tilapia fingerlings (5.65 cm and 2.61 g) were used. Nine aquariums (55 L) were used. Six containers were installed in each aquarium, where one prawn was stocked per container (32 prawn/m2), and in the remaining water, tilapia was stocked at densities of 100, 200 and 300 fish/m3. Balanced feed was used. The daily ration for prawns was 6% and for tilapia, it was 5% of the total biomass. The experiment lasted 90 days. Results. In prawns, the length (6.46 cm), weight (9.37 g), percentage gains in length (10.01% at 10.45%) weight (19.24% a 25.41%), and survival (88.89% to 94.44%) were similar (p<0.05) between treatments. The effect of molting death syndrome is discussed. In tilapia, the length (9.25 cm), weight (12.90 g), absolute growth rate (0.040 cm/day; 0.114 g/day), specific growth rate (0.55% length/day; 1.759% weight/day) and percentage gain (64.21%; 389.48%) were greater (p<0.05) at 100 and 200 fish/m3. Tilapia survival was similar (86.11%) between treatments. Conclusions. Prawn growth and survival were affected by molt death syndrome but not by the presence of tilapia in the system. In contrast, greater growth of tilapia was obtained with 100 fish/m3, although survival was similar between treatments.
Article visits 1363 | PDF visits
Downloads
- Fitzsimmons KM, Shahkar E. Tilapia-Shrimp polyculture. In: Perschbacher PW, Stickney RR (Eds). Tilapia in intensive co-culture. Wiley Blackwell: EU; 2017. https://www.doi.org/10.1002/9781118970652.ch10
- New MB, Valenti WC. Tilapia-Macrobrachium polyculture. In: Perschbacher PW, Stickney RR. (Eds) Tilapia in intensive co-culture. Wiley Blackwell: EU; 2017. https://www.doi.org/10.1002/9781118970652.ch11
- Schmalenbach I, Buchholz F, Franke HD, Saborowski R. Improvement of rearing conditions for juvenile lobster (Homarus gammarus) by co-culturing with juvenile isopods (Idotea emarginata). Aquac. 2009; 289(3-4):297-303. https://www.doi.org/10.1016/j.aquaculture.2009.01.017
- Rohmana D, Surawidjaja EH, Sukenda S, Ekasari J. Water quality and production performance of catfish-prawn co-culture with organic carbon source addition. Aquacult Int. 2015; 23:267-276. https://www.doi.org/10.1007/s10499-014-9814-2
- Tidwell JH, Coyle SD, Bright LA. Polyculture of Nile tilapia, Oreochromis niloticus, either confined in cages or unconfined in freshwater prawn, Macrobrachium rosenbergii, ponds. J World Aquac Soc. 2010; 41(4):616-625. https://www.doi.org/10.1111/j.1749-7345.2010.00402.x
- Tesfahun A, Temesgen M. Food and feeding habits of Nile tilapia Oreochromis niloticus (L.) in Ethiopian water bodies: A review. Int J Fish Aquat Stud. 2018; 6(1):43-47. http://www.fisheriesjournal.com/archives/2018/vol6issue1/PartA/5-6-54-506.pdf
- Nunoo FKE, Asase A. Comparative growth performance of Oreochromis niloticus (Linnaeus, 1758) in cages at different stocking densities. Int J Fish Aquat Stud. 2017; 5(4):279-283. http://www.fisheriesjournal.com/archives/2017/vol5issue4/PartD/5-4-4-982.pdf
- Araujo G, Rodrigues J, Da Silva J, Farias W. Cultivo da tilápia do Nilo Oreochromis niloticus em tanques-rede circulares em diferentes densidades de estocagem. Biosci J. 2010; 26(3):428-434. http://www.seer.ufu.br/index.php/biosciencejournal/article/view/7080
- Abdel-Tawwab M. Effects of dietary protein levels and rearing density on growth performance and stress response of Nile tilapia, Oreochromis niloticus (L.). Int Aquat Res. 2012; 4(3):1-12. https://www.doi.org/10.1186/2008-6970-4-3
- Yakubu A, Obi A, Okonji V, Ajiboye O, Adams T, Olaji E, et al. Growth performance of Nile tilapia (Oreochromis niloticus) as affected by stocking density and feed types in water flow through system. World J Fish Marine Sci. 2012; 4(3):320-324. http://www.doi.org/10.5829/idosi.wjfms.2012.04.03.6230
- Shubha M, Reddy S. Effect of stocking density on growth, maturity, fecundity, reproductive behaviour and fry production in the mouth brooding cichlid Oreochromis mossambicus (Peters). Afr J Biotechnol. 2011; 10(48):9922-9930. http://dx.doi.org/10.5897/AJB11.697
- Moscoso V. Catálogo de crustáceos decápodos y estomatópodos del Perú. Bol Inst Mar Perú. 2012; 27(1-2):8-207. http://biblioimarpe.imarpe.gob.pe/handle/123456789/2190
- Wasiw J, Yépez V. Evaluación poblacional del camarón Cryphiops caementarius en ríos de la costa sur del Perú. Rev Inv Vet Perú. 2015; 26(2):166-181. http://dx.doi.org/10.15381/rivep.v26i2.11103
- Ministerio de la Producción. Anuario estadístico pesquero y acuícola 2017. La actividad productiva del sector en números, Perú. 2018. http://ogeiee.produce.gob.pe/images/Anuario/Pesca_2017.pdf
- Reyes W. Effect of culture container on the survival and growth of male Cryphiops caementarius in individualized systems. Revista Bio Ciencias. 2016; 3(4):311-325. https://www.doi.org/10.15741/revbio.03.04.06
- Manor R, Segev R, Pimenta M, Aflalo ED, Sagi A. Intensification of redclaw crayfish Cherax quadricarinatus culture II. Growout in a separate cell system. Aquacult Eng. 2002; 26(4):263-276. https://www.doi.org/10.1016/S0144-8609(02)00035-3
- Drengstig A, Bergheim A. Commercial land-based farming of European lobster (Homarus gammarus L.) in recirculating aquaculture system (RAS) using a single cage approach. Aquacult Eng. 2013; 53:14–18. https://www.doi.org/10.1016/j.aquaeng.2012.11.007
- Méndez M. Claves de identificación y distribución de los langostinos y camarones (Crustacea: Decapoda) del mar y ríos de la costa del Perú. Bol Inst Mar Perú. 1981; 5:1-169. http://biblioimarpe.imarpe.gob.pe/handle/123456789/1028
- Reyes W, Terrones S, Baltodano I. Effects chelipeds regeneration in molting and growth of male Cryphiops caementarius Molina 1782 (Decapod, Palaemonidae). Revista Bio Ciencias. 2017; 4(4): 1-18. http://dx.doi.org/10.15741/revbio.04.04.05
- Cornejo J, Pérez L, Reyes W. Effect of Saccharomyces cerevisiae yeast in the diet of male shrimp Cryphiops caementarius (Crustacea, Palaemonidae) on total and differential hemocytes count. Revista Bio Ciencias. 2015; 3(3):173-186. http://dx.doi.org/10.15741/revbio.03.03.04
- Pandit NP, Nakamura M. Effect of high temperature on survival, growth and feed conversion ratio of Nile tilapia, Oreochromis niloticus. Our Nature. 2012; 8(1):3219-224. https://doi.org/10.3126/on.v8i1.4331
- López-Gómez C, Ponce-Palafox T, Castillo-Vargasmachuca S, Puga-López D, Castillo-Campo LF, García-Ulloa M. Evaluation of two mix-cultures of white shrimp (Litopenaeus vannamei) with red tilapia hybrid and spotted rose snapper (Lutjanus guttatus) in intensive indoor brackish water tanks. Lat Am J Aquat Res. 2017; 45(5):922-929. http://www.doi.org/10.3856/vol45-issue5-fulltext-7
- Juárez-Rosales J, Ponce-Palafox T, Román-Gutiérrez AD, Otazo-Sánchez OM, Pulido-Flores G, Castillo-Vargasmachuca SG. Effects of White shrimp (Litopenaeus vannamei) and tilapia nilotica (Oreochromis niloticus var, Spring) in monoculture and co-culture systems on water quality variables and production in brackish low-salinity water earthen ponds during rainy and dry seasons. Span J Agric Res. 2019; 17(3): e0605. https://www.doi.org/10.5424/sjar/2019173-14938
- Wasiw J, Yépez V. Evolución de la condición poblacional del camarón Cryphiops caementarius en el río Cañete (2000-2015). Rev Inv Vet Perú. 2017; 28(1):13-32. http://dx.doi.org/10.15381/rivep.v28i1.12942
- Acosta A, Quiñones D, Reyes W. Efecto de dietas con lecitina de soya en el crecimiento, muda y supervivencia de machos del camarón de río Cryphiops caementarius (Crustacea: Palaemonidae). Sci Agropecu. 2018; 9(1):143-151. http://www.doi.org/10.17268/sci.agropecu.2018.01.15
- Terrones S, Reyes W. Efecto de dietas con ensilado biológico de residuos de molusco en el crecimiento del camarón Cryphiops caementarius y tilapia Oreochromis niloticus en cocultivo intensivo. Sci Agropecu. 2018; 9(2):167-176. http://www.doi.org/10.17268/sci.agropecu.2018.02.01
- Ramírez M, Cántaro R, Reyes W. Growth and survival of males of Cryphiops caementarius (Palaemonidae) with diets supplemented with common salt. Lat Am J Aquat Res. 2018; 46(2):469-474. http://www.doi.org/10.3856/vol46-issue2-fulltext-22
- Reyes W. El síndrome de la ecdisis incompleta en machos adultos de Cryphiops caementarius (Crustacea: Palaemonidae) y sus consecuencias en cultivo intensivo. Rev Inv Vet Perú. 2018; 29(1):68-374. http://dx.doi.org/10.15381/rivep.v29i1.14200
- Yasuda CI, Matsuo K, Wada S. Rapid regeneration of the major cheliped in relation to its function in male-male contests in the hermit crab Pagurus middendorffii. Plankton Benthos Res. 2014; 9(2):122-131. https://www.doi.org/10.3800/pbr.9.122
- Islam ML, Siddiky MNSM, Yahya K. Growth, survival and intactness of green mud crab (Scylla paramamosain) broodstock under different captive grow out protocols. SAARC J Agri. 2018; 16(1):169-180. http://dx.doi.org/10.3329/sja.v16i1.37432
- Daniels CL, Wills B, Ruiz-Perez M, Miles E, Wilson RW, Boothroyd D. Development of sea-based container culture for rearing European lobster (Homarus gammarus) around South West England. Aquac. 2015; 448:186-195. http://dx.doi.org/10.1016/j.aquaculture.2015.05.026
- Teodósico R, Engrola S, Colen R, Masagounder K, Aragão C. Optimizing diets to decrease environmental impact of Nile tilapia (Oreochromis niloticus) production. Aquacult Nutr. 2019; 26(2): 422-431. http://www.doi.org/10.1111/anu.13004
- Liu Y, Wen JJ, Ning LJ, Jiao JG, Qiao F, Chen LQ, et al. Comparison of effects of dietary-specific fatty acids on growth and lipid metabolism in Nile tilapia. Aquacult Nutr. 2019; 25(4):862-872. https://doi.org/10.1111/anu.12906
- Bessa AP, Borges CMS, Silva F, Henry-Silva GG. Polyculture of Nile tilapia and shrimp at different stocking densities. R Bras Zootec. 2012; 41(7):1561-1569. http://dx.doi.org/10.1590/S1516-35982012000700002
- Costa ÂAP, Roubach R, Dallago BSL, Bueno GW, McManus C, Bernal FEM. Influence of stocking density on growth performance and welfare of juvenile tilapia (Oreochromis niloticus) in cages. Arq Bras Med Vet Zootec. 2017; 69(1):243-251. http://dx.doi.org/10.1590/1678-4162-8939
- Tidwell JH, Coyle SD, Bright LA. Polyculture of Nile tilapia, Oreochromis niloticus, either confined in cages or unconfined in freshwater prawn, Macrobrachium rosenbergii, ponds. J World Aquac Soc. 2010; 41(4):616-625. https://www.doi.org/10.1111/j.1749-7345.2010.00402.x
- Osofero S, Otubusin S, Daramola J. Effect of stocking density on tilapia (Oreochromis niloticus Linnaeus 1757) growth and survival in bamboo-net cages trial. Afr J Biotechnol. 2009; 8(7):1322-1325. https://www.ajol.info/index.php/ajb/article/view/60113/48369
- Hernández-Vergara MP, Cruz-Ordóñez SB, Pérez-Rostro CI, Pérez-Legaspi A. Polyculture of crayfish (Procambarus acanthophorus) and Nile tilapia (Oreochromis niloticus) as a strategy for sustainable water use. Hidrobiológica. 2018; 28(1):11-15. https://www.doi.org/10.24275/uam/izt/dcbs/hidro/2018v28n1/HernandezV
- Mondal A, Bhattacharya S, Mitra A, Sundaray JK, Mohanty RK. Performance evaluation of mud crab Scylla olivacea (Herbst, 1896) coculture with different fish species in confined brackishwater ponds. Aquac. 2020; 522:735125. https://doi.org/10.1016/j.aquaculture.2020.735125























