Survival, development, and growth of Penaeus vannamei larvae fed on traditional and non-traditional diets shrimp larvae feeding
Supervivencia, desarrollo y crecimiento de larvas de Penaeus vannamei alimentadas con dietas tradicionales y no-tradicionales

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
Show authors biography
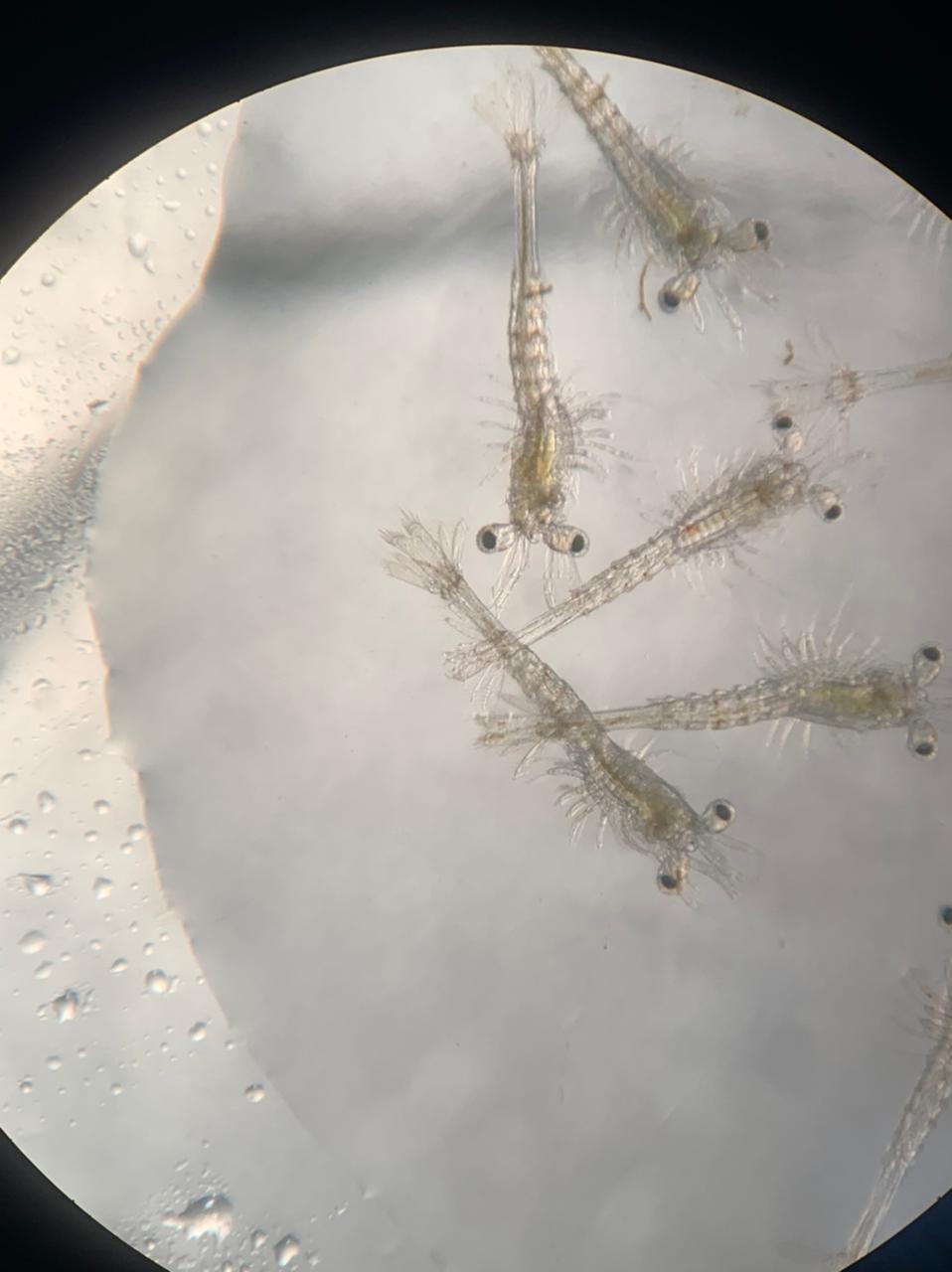
Objetive. The effect of traditional diets (Thalassiosira weissflogii and Artemia nauplii) and two other alternatives (not traditional) based on microalgae with rotifers were evaluated on the survival, development and growth from nauplii V (NV) larvae until postlarvae (PL1) of Penaeus vannamei. Materials and methods. Sixteen replicates (experimental units) were applied for each of the three diets used. The larvae were placed in 12 L containers at 35 psu, 30°C and a density of 200larvae/L. At the beginning, every 24 h and the end of the experiment (PL1: day 8), samples of larvae were obtained to determine survival, development and growth (length and weight). A one-way ANOVA was applied to the data obtained. Results. Diet had no influence (p>0.05) on survival and weight. Survival ranged from 30.4% (alternative diet B) to 28.5% (traditional diet A). The lowest development, length and weight at PL1 was found with the traditional diet (6.71; 3.53 mm; 58.37 µm/organism) compared with the alternative diets supplied B and C (6.86-6.76; 3.79-3.82 mm; 60.7-65.0 µm/organism. Conclusions. Non-traditional alternative diet (B and C) composed of rotifers was the best diet for larval survival, development and growth.
Article visits 452 | PDF visits
Downloads
- Arzola GJ, Piña VP. Nieves SM, Medina JM. Supervivencia de postlarvas de camarón blanco Litopenaeus vannamei a diferentes salinidades y temperaturas. Rev MVZ Cordoba. 2013; 18(Supl):3618-3625. https://doi.org/10.21897/rmvz.127
- Medina-Jasso M, Arzola-González JF, Piña-Valdez P, Nieves-Soto M. Effect of the diet traditional and non-traditional on the respiration and excretion in larvae of white shrimp Litopenaeus vannamei. Rev MVZ Cordoba. 2015; 20(Supl):4917-4928. https://doi.org/org/10.21897/rmvz.7
- Bermudes-Lizárraga J, Nieves-Soto M, Medina-Jasso A, Piña-Valdez P. Efecto de temperatura y salinidad sobre la supervivencia y desarrollo larval de Litopenaeus vannamei. Rev MVZ Cordoba. 2017; 22(2):5844-5853. https://doi.org/10.21897/rmvz.1022
- Gallardo P, Martínez G, Palomino G, Paredes A, Gaxiola G, Cuzon G, et al Replacement of Artemia franciscana nauplii by microencapsulated diets: effect on development, digestive enzymes, and body composition of white shrimp, Litopenaeus vannamei, larvae. J World Aquac Soc. 2013; 44(2):187–197. https://doi.org/10.1111/jwas.12031
- Varela-Mejias A, Varela-Moraga T. La camaronicultura como fuente sustentable de alimentos de origen animal. Logros, retos y oportunidades. Eco Des Sos. 2019; 1(1):1-12. https://revistas.ulatina.ac.cr/index.php/ecologia/article/view/306
- Jamali H, Ahmadifard N, Abdollahi D. Evaluation of growth, survival and body composition of larval white shrimp (Litopenaeus vannamei) fed the combination of three types of algae. Int Aquat Res. 2015; 7:115-122. https://doi.org/10.1007/s40071-015-0095-9
- Bermudes-Lizárraga JF, Nieves-Soto M, Medina-Jasso MA, Román-Reyes JC, Flores-Campaña LM, Ortega-Salas AA, et al Efecto de la temperatura y salinidad en el crecimiento larval de Litopenaeus vannamei. Rev Biol Mar Oceanogr. 2017; 52(3):611-615. http://dx.doi.org/10.4067/S0718-19572017000300016
- López-Elías JA, Nevárez-Pineli ML, Aguirre-Hinojoza E, Martínez-Córdova LR, Valdez-Holguin JE. Estudio económico de laboratorios de producción de larvas de Litopeneaus vannamei (camarón blanco). Biotecnia 2013; 15(1):19-24. https://biotecnia.unison.mx/index.php/biotecnia/article/view/131
- García N, López-Elías JA, Miranda A, Martínez-Porchas M, Huerta N, García A. Effect of salinity on growth and chemical composition of the diatom Thalassiosira weissflogii at three culture phases. Lat Am J Aquat Res 2012; 40(2):435-440. https://doi.org/10.3856/vol40-issu2-fulltext-18
- Ruíz-Guzmán JA, Jiménez-Velásquez CA, Gomes-Romero C, Prieto-Guevara MJ. Experimental culture of Cyclopina sp with differents microalgae’s species. Rev Colomb Cienc Pecu. 2012; 25(1):97-105. https://www.proquest.com/docview/1247121829
- Jeyaraj N, Santhanam P. Influence of algal diet on population density,egg production and hatching succession of the calanoid copepod, Paracalanus parvus (Claus, 1863). J Algal Biomass Utln. 2013; 4(1):1-8. http://storage.unitedwebnetwork.com/files/521/2824198906703a2f941bf82d0396f1c0.pdf
- Rojo-Cebreros AH, Román-Reyes JC, Rodríguez-Montes de Oca GA, Nieves-Soto M, Piña-Valdez P, Medina-Jasso MA. Balance energético del rotífero Brachionus rotundiformis Tschugunoff 1921, alimentado con cuatro especies de microalgas. Univ Cienc. 2012; 28(3):231-244. https://doi.org/10.19136/era.a28n3.12
- Román-Reyes JC, Castañeda-Rodríguez DO, Castillo-Ureta H, Bojórquez-Domínguez R, Rodríguez-Montes de Oca GA. Dinámica poblacional del rotífero Brachionus ibericus aislado de estanques para camarón, alimentado con diferentes dietas. Lat Am J Aquat Res 2014; 42(5):1159-1168. http://doi.org/10.3856/vol42-issue5-fulltext-19
- Arreguin-Rebolledo U, Sarma N, Rodríguez-Montes de Oca GA, Monroy-Dosta MC, Tello-Ballinas JA, Sarma SSS, Román-Reyes JC. The potential use of the euryhaline rotifer Proales similis for larval rearing of the freshwater pike silverside Chirostoma estor estor. Aquacult 2021; 534:1-8. https://doi.org/10.1016/j.aquaculture.2020.736246
- Ruiz-Toquica JS, Becerra-Real LM, Villamil-Díaz LM. Evaluación del efecto de Bacillus firmus C101 en el crecimiento de poslarvas de Litopenaeus vannamei Boone (camarón blanco) y Brachionus plicatilis s.s. Müller (rotífero). Bol Invest Mar Cost 2020; 49(1):63-80. https://doi.org/10.25268/bimc.invemar.2020.49.1.774
- Piña P, Nieves M, Voltolina D, Chavira-Ortega C. Crecimiento, desarrollo y supervivencia de mysis de Litopenaeus vannamei alimentadas con nauplios de Artemia y con el rotífero Brachionus plicatilis. Rev Invest Mar 2004; 25(3):245-251.
- Nieves-Soto M, Lozano-Huerta R, López-Peraza DJ, Medina-Jasso MA, Hurtado-Oliva MA, Bermudes-Lizárraga JF. Effect of the enrichment time with the tuna orbital oil emulsion on the fatty acids profile of juveniles of Artemia franciscana. Aquac Fish 2021; 6(1):69-74. https://doi.org/10.1016/j.aaf.2020.03.008
- Piña-Valdez P, Arzola-González JF, Nieves-Soto M, Medina-Jasso M. Efecto combinado de temperatura y salinidad en el consumo de oxígeno en postlarvas de camarón blanco Litopenaeu vannamei. Bol Inst Pesca 2015; 41(1):89-101. https://intranet.institutodepesca.org/41_1_89-101.pdf
- Zar JH. Biostatistical analysis. Upper Saddle River, USA: Prentice-Hall Inc; 2010.
- Coelho RTI, Yasumaru FA, Passos MJACR, Gomes V, Lemos D. Energy budgets for juvenile Pacific whiteleg shrimp Litopenaeus vannamei fed different diets. Braz J Oceanogr 2019; 67:1-8.
- http://dx.doi.org/10.1590/S1679-87592019024306701
- Rodríguez Canché LG, Maldonado-Montiel TDNJ, Carrillo-Navarro LA. Calidad biológica y bioquímica de la población de Artemia (Anostraca: Artemiidae) localizada en las salinas de Real de Salinas, Calkiní, Campeche, México. Rev Biol Trop 2006; 54(4):1283-1293. https://doi.org/10.15517/rbt.v54i4.3104
- Campaña-Torres A, Martínez-Córdova LR, Martínez-Porchas M, López-Elías JA, Porchas-Cornejo MA. Productive response of Nannochloropsis oculata, cultured in different media and their efficiency as food for the rotifer Brachionus rotundiformis. J Exp Bot 2012; 81:45-50. http://www.revistaphyton.fund-romuloraggio.org.ar/vol81/6-CAMPANA-TORRES.pdf
- Gelabert R, Brito R, Gaxiola G, Castro T, Rosas C. Efecto de nauplios de "Artemia franciscana" enriquecidos sobre el crecimiento, supervivencia y resistencia al estrés de postlarvas (PL5-PL40) de "Litopenaeus vannamei" (Boone, 1931). Univ Cienc 2008; 24(1):29-39. http://dx.doi.org/10.19136/era.a24n1.277
- Seychelles LH, Happe S, Palacios E, Ludwig M, Hollmer S, Ehlers RU, et al Successful rearing of whiteleg shrimp Litopenaeus vannamei larvae fed a desiccation-tolerant nematode to replace Artemia. Aquac Nutr 2018; 24:903-910. https://doi.org/10.1111/anu.12626
- Ordóñez-Mejía CA, Galarza-Mora WG, Quizhpe-Cordero P, Quijije-López LJ. Efecto de la combinación de alimento artificial y biomasa de Artemia sp en cría intensiva de postlarvas de Litopenaeus vannamei. Dom Cien 2021; 7(2):1167-1189. https://dominiodelasciencias.com/ojs/index.php/es/article/view/1854
- Cobo ML, Wouters R, Wille M, Sonnenholzner S, Sorgeloos P. Evaluation of frozen umbrella‐stage Artemia as first animal live food for Litopenaeus vannamei (Boone) larvae. Aquac Res 2015; 46:2166-2173. https://doi.org/10.1111/are.12372
- Martínez-Córdova LF, Martínez-Porchas M, López-Elías JA, Enríquez-Ocaña LF. Uso de microorganismos en el cultivo de crustáceos. Biotecnia 2014; 16(3):50-55. https://biotecnia.unison.mx/index.php/biotecnia/article/view/141
- Rodríguez EO, López-Elías JA, Aguirre-Hinojosa E, Garza-Aguirre MDC, Constantino-Franco F, Miranda-Baeza A, et al Evaluation of the nutritional quality of Chaetoceros muelleri schutt (Chaetocerotales: Chaetocerotaceae) and Isochrysis sp. (Isochrysidales: Isochrysidaceae) grown outdoors for the larval development of Litopenaeus vannamei (Boone, 1931) (Decapoda: Penaeidae). Arch Biol Sci 2012; 64(3):963-970. https://doi.org/10.2298/ABS1203963R
- Yoshimatsu T, Hossain MA. Recent advances in the high-density rotifer culture in Japan. Aquacult Int 2014; 22:1587-1603. https://doi.org/10.1007/s10499-014-9767-5